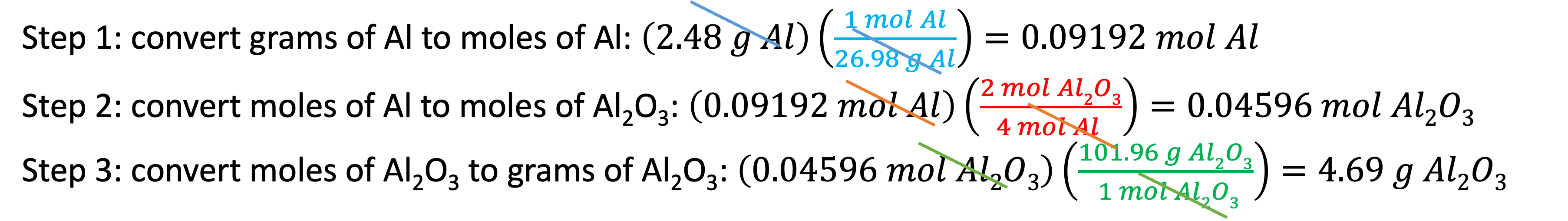In chemistry, we do dimensional analysis quite often, therefore, it is important to learn some skills so that you can complete tasks quickly and accurately. First of all, a conversion factor is the equivalent of two quantities expressed in a fraction. For example, if x quantity of A is equivalent to y quantity of B, then the conversion factor will be either x ⁄y for converting the amount of B to A, or
(a) volume ↔ volume or mass ↔ mass conversion.
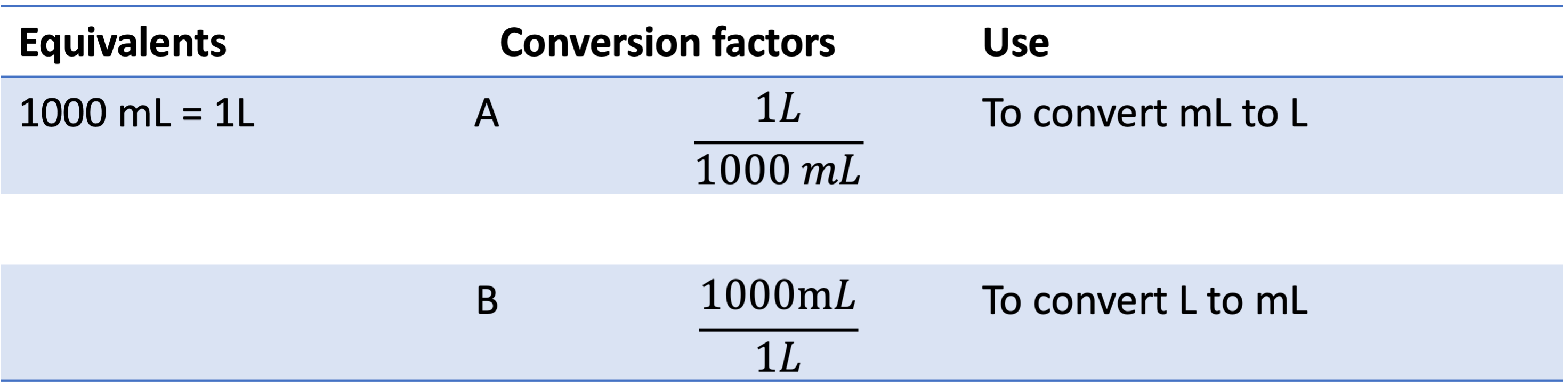
These calculations often are related to different units. The following table shows two conversion factors for converting volume between L and mL. The equivalent is 1000 mL = 1.0 L. The conversion factor A is used to convert mL to L whereas factor B is used to convert L to mL. You can apply the same strategy for converting kg to g, m to nm, m to inch, etc.
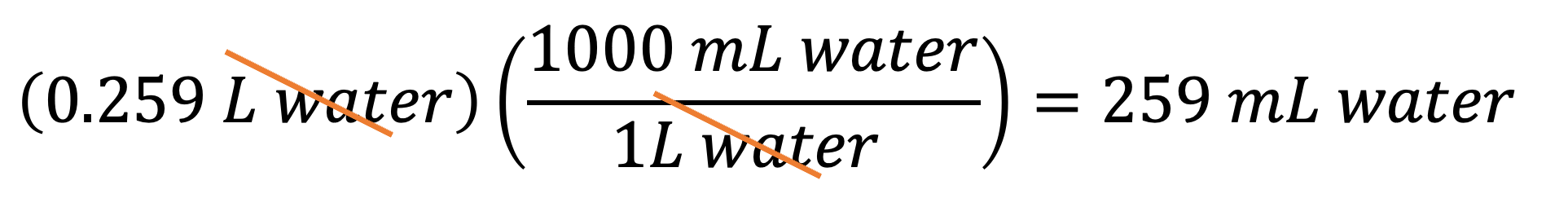
 Example 1: If you have 0.259 L of water, what is its volume in mL? You can multiply 0.259 L by the conversion factor B to get your answer:
Example 1: If you have 0.259 L of water, what is its volume in mL? You can multiply 0.259 L by the conversion factor B to get your answer:
 Example 2: If you have 56.3 mL of water, what is its volume in L? You can multiply 56.3 mL by the conversion factor A to get your answer:
Example 2: If you have 56.3 mL of water, what is its volume in L? You can multiply 56.3 mL by the conversion factor A to get your answer:
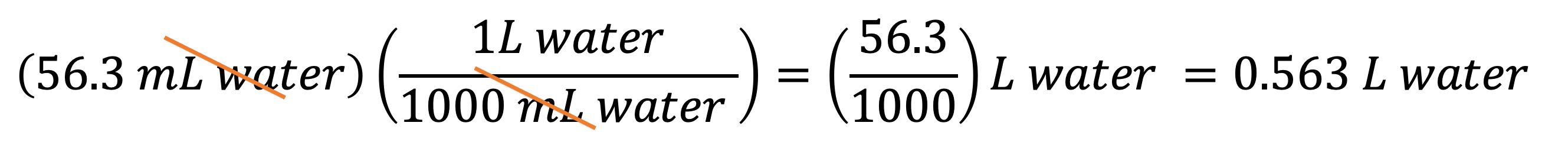
(b) gram ↔ mole conversion.
You need to use the molar mass of an atom or a molecule as a conversion factor. Let's use NaCl as an example. Its molar mass is 58 g/mol, which means 1 mol NaCl = 58 g NaCl. Therefore, there are two conversion factors for converting quantities between grams and moles. Keep in mind that molar mass is numerically equal to the amu of the atom or the molecule, but the unit is g/mol.
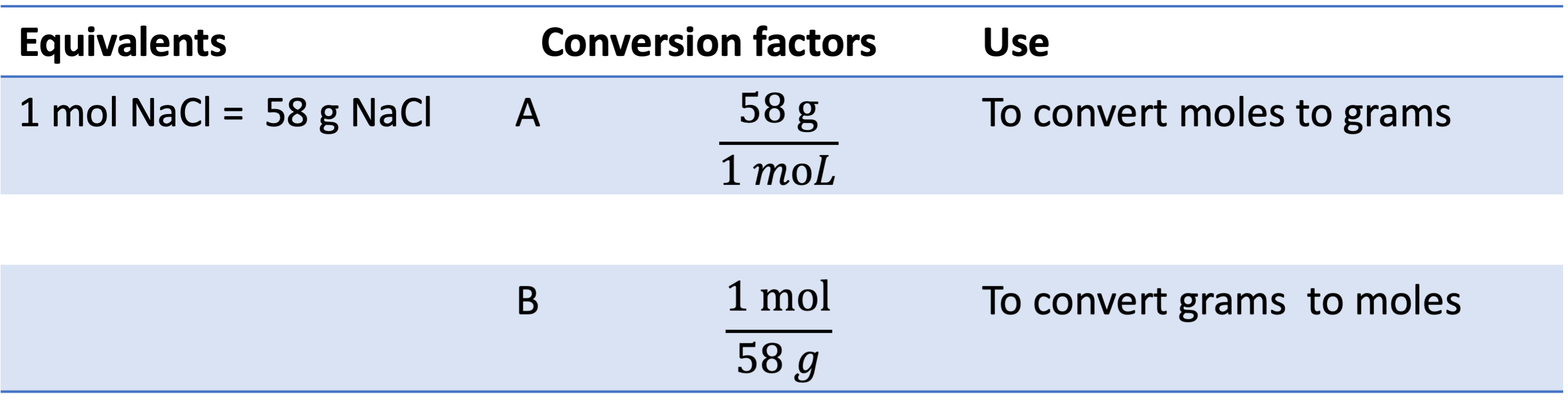 Example 3: If you have 0.125 moles of NaCl, what is its mass in g? You can multiply 0.125 L by conversion factor A to get your answer:
Example 3: If you have 0.125 moles of NaCl, what is its mass in g? You can multiply 0.125 L by conversion factor A to get your answer:
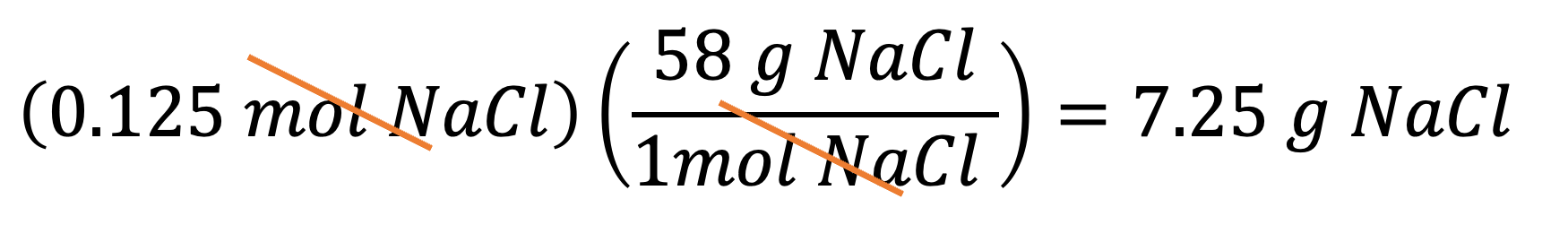 Example 4: If you have 110 g of NaCl, how many moles of NaCl are in it? You can multiply 110 g by the conversion factor B to get your answer:
Example 4: If you have 110 g of NaCl, how many moles of NaCl are in it? You can multiply 110 g by the conversion factor B to get your answer:
![]()
(c) gram ↔ volume conversion.
You need to use density to do this type of conversion. Let's use ethanol as an example. Ethanol has a density of 0.789 g/mL. which means 0.789 g ethanol = 1 mL ethanol. Therefore, there are two possible conversion factors as shown in the following table. Most times, the desnity will be provided if you are expected to perform such a conversion. Please be aware that the unit of density is not always g/mL, it could be g/L, kg/L, etc. In that case, you can use the method in examples 1 and 2 to convert the unit to g/mL or the one that is the same as the given quantity.
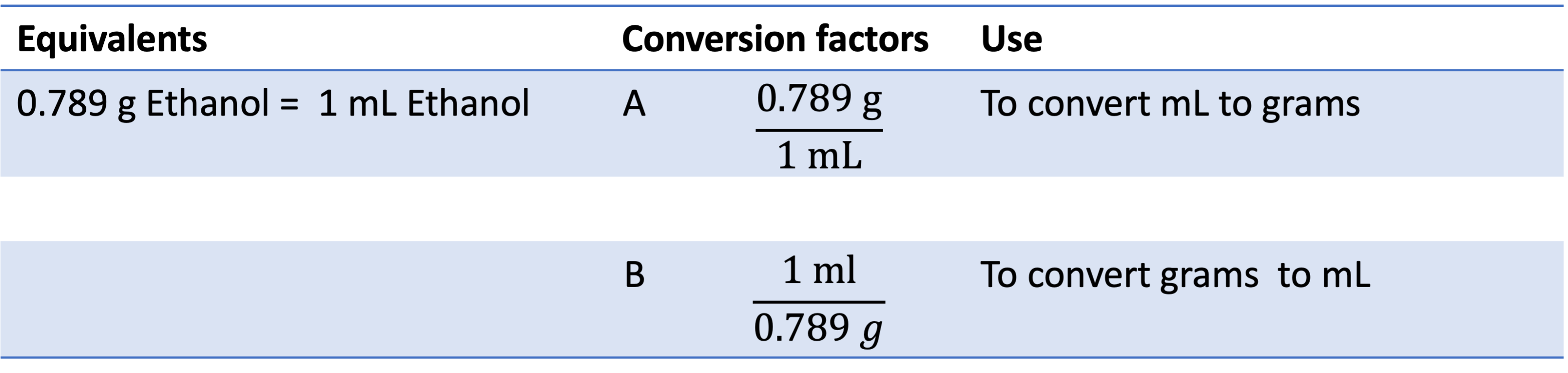 Example 5: If you have 125 mL of ethanol, what is its mass in g? You can multiply 125 mL by conversion factor A to get your answer:
Example 5: If you have 125 mL of ethanol, what is its mass in g? You can multiply 125 mL by conversion factor A to get your answer:
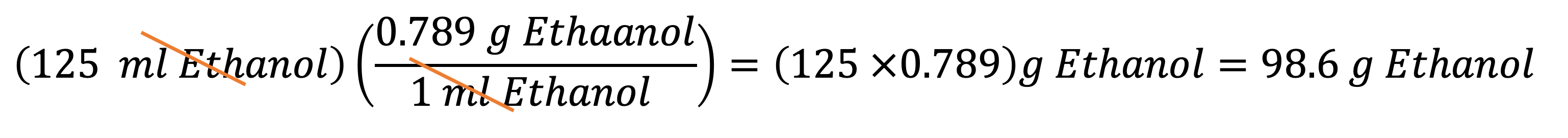 Example 6: If you have 85.2 g of ethanol, what is its volume in mL? You can multiply 85.2 g by the conversion factor B to get your answer:
Example 6: If you have 85.2 g of ethanol, what is its volume in mL? You can multiply 85.2 g by the conversion factor B to get your answer:
![]()
(d) mole ↔ mole conversion.
This type of conversion often relates to a chemical reaction. The conversion factor is the ratio of two coefficients. For the following balanced chemical equation, you can have several conversion factors depending upon which two chemicals are involved in your calculations.
2 O2 (g) + 4 Al (s) → 2 Al2O3 (s)
Two conversion factors that involve O2 and Al2O3 are shown in the following table.
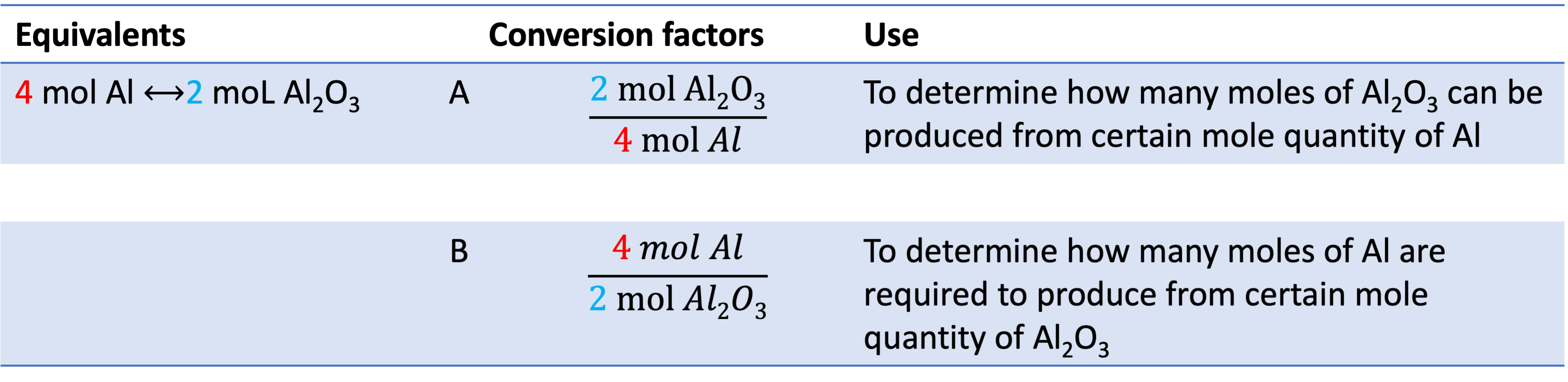 Example 7: If you have 0.24 moL of Al, how many moles of Al2O3 will be produced? You can multiply 0.24 moL by the conversion factor A to get your answer:
Example 7: If you have 0.24 moL of Al, how many moles of Al2O3 will be produced? You can multiply 0.24 moL by the conversion factor A to get your answer:
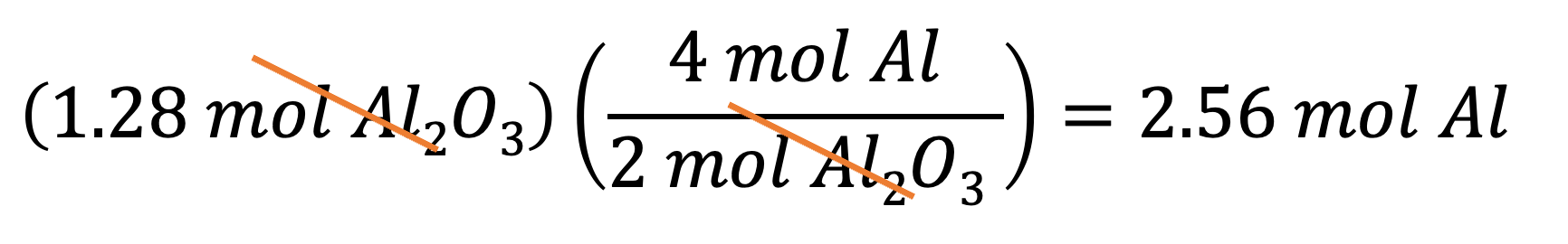
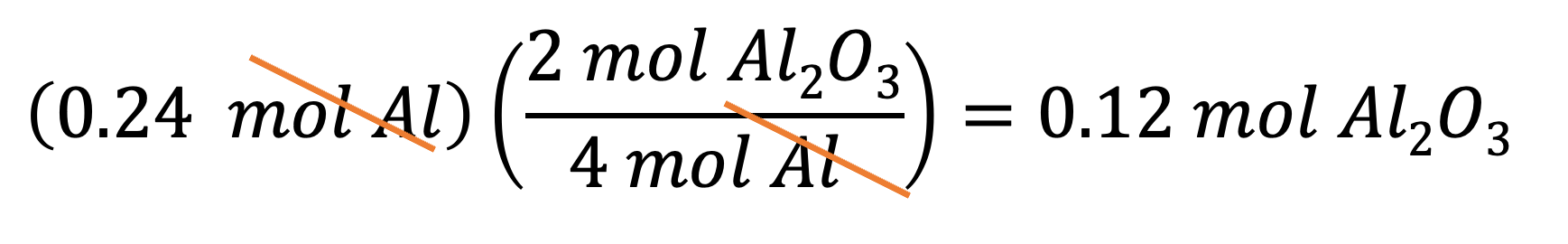 Example 8: If you want to produce 1.28 mol of Al2O3, then how many moles of Al do you need? You can multiply 1.28 mol by the conversion factor B to get your answer:
Example 8: If you want to produce 1.28 mol of Al2O3, then how many moles of Al do you need? You can multiply 1.28 mol by the conversion factor B to get your answer:
In most cases, the conversion is not as straightforward as we show in previous examples. It requires several conversion steps, which means it requires several conversion factors. What you can do is to multiply the given quantity by all conversion factors. I am not a big fan of this method, even though the method is widely taught in chemistry classes.
Example 9: If you have 125 mL of ethanol, how many moles of ethanol molecules are in it? In this case, you will use the conversion factor A in example 5 to convert volume (in mL) to mass (in g), then multiply the result by the second conversion factor (1 mol ethanol)/(47.07g ethanol) to get the final answer:
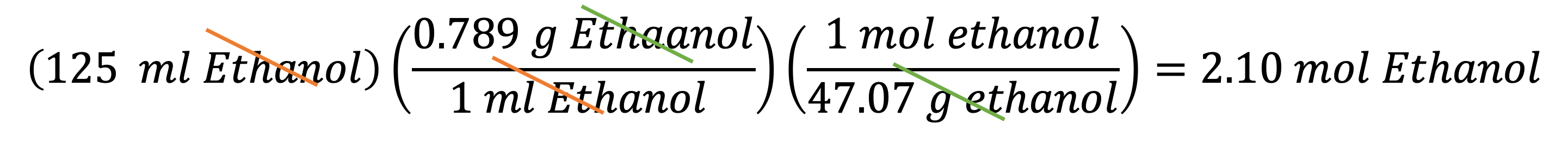
Example 10: How many grams of Al2O3 will be produced when 2.48 g Al reacts completely with an excess of O2? We will use three conversion factors: the molar mass of Al (1 mol/26.98 g Al) to convert 2.48 g Al to its moles, the molar ratio ( 2 mol Al2O3/4 mol Al) to convert moles of Al to moles of Al2O3, then the molar mass of Al2O3 (101.96 g Al2O3/1 mol Al2O3) to convert moles of Al2O3 to grams of Al2O3
2 O2 (g) + 4 Al (s) → 2 Al2O3 (s)
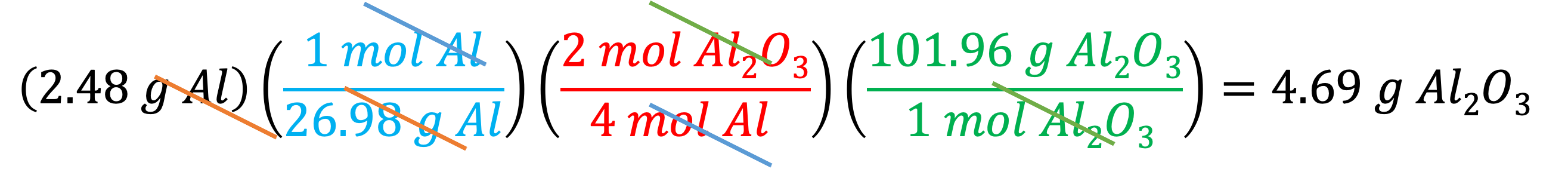 My favorite method is to complete this task in three steps, each using a single conversion factor as I show below.
My favorite method is to complete this task in three steps, each using a single conversion factor as I show below.