Welcome to PERIYANNAN RESEARCH GROUP |
 |
| Home | Gopal R Periyannan | Research | Teaching |
Group Members
Publications
Protease Links
Other Links
Protein Instability Index Calculator
| Research |
|
One third of all known proteins are
metalloproteins. The number of important enzymes containing metal
ions in their active site is increasing; which thereby underscores the
significance of the role played by metalloenzymes in biology and
medicine. Our research focuses on the structure and the
mechanistic aspects of Zn-metalloproteases in order to understand their
physiological function and their role in disease development.
Zinc Metalloproteases
GCPII is a 750 residue,
dimeric, transmembrane,
Zn-metalloprotease expressed in the central nervous system and other
types of
tissues. The exact roles played by the members of GCPII class of
proteins
in different types of tissues, other than neuronal tissues, are not
well
understood. GCPII is expressed in large amounts in prostate cancer
tissue and in the newly forming blood vessels of most
solid
tumors hence thought to be contributor of cancer progression. In
addition to
the peptidase activity, GCPII is predicted to have cell signaling
properties. Although
considerable structural characterization has been carried out on GCPII,
including crystal structures of the extracellular domains, the
mechanism of
catalysis remains unproved. A precise understanding of the steps
involved in
the catalytic mechanism of NAAG hydrolysis will immensely increase the
scope of
synthesizing novel drugs, including candidates that can cross the
blood-brain
barrier. Further, it is essential to probe for any signaling event
associated
with substrate binding or catalysis to reveal any other roles of GCPII.
Such
studies are hampered by the absence full-length GCPII or a homolog.
Currently
available GCPII expression systems provide only the Extracellular
portion of
the protein, and that in small quantity and at a great expense. We
propose to
overcome this hurdle by studying a suitable model protein for GCPII in
an
appropriate model organism.
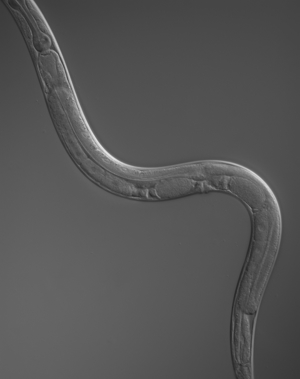 GCPII Model Protease from Caenorhabditis elegans
GCPII Model Protease from Caenorhabditis elegansWe explored the genomic databases and
identified a transmembrane Zn metalloprotease in nematode C.
elegans with remarkable structural homology to GCPII, including
the M28 family
of Zn protease
domain, transferring receptor like dimerization domain and potential
for glycosylation.
These structural similarities between the metalloprotease from S.
pombe and GCPII prompt us to
propose this putative protein as a suitable model to study
structure-function
relationships and the hydrolytic mechanism of GCPII class of proteins.
We use spectroscopic
techniques to probe
the Zn(II) mediated catalytic mechanism of GCPII.
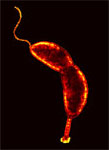 Metalloprotease
from Caulobacter crescentus Metalloprotease
from Caulobacter crescentusAs
a part of our investigation of Zn-metalloproteases and their roles in
disease
development, we have cloned and successfully tested the expression of a
Zn-metalloprotease
from Caulobacter crescentus, a gram
negative bacterium, which is an important model organism for study of
the cell
cycle and cellular differentiation. The cloned metalloprotease shares
amino
acid sequence similarity with GCPII. Information gathered on the active
site and the
catalytic mechanism of this metalloprotease is expected to help
understand the catalytic mechanism of GCPII.
|
| Biofuel Microbial Biomass Conversion Biofuels hold
the promise to supplement our
future energy needs in a renewable and sustainable manner. Recently
there have
been reports of conversion of plant-derived biomass into fuel quality
carbon
molecules through the combination of biological and chemical means. The
feedstock molecules for these conversion processes have been mostly
monosaccharides
derived from plant polysaccharides. There is a continued search for an
efficient
enzyme system which could breakdown the unusual plant polysaccharides
such as
cellulose, pectin and xylan in a cost effective manner into their sugar
makeup.
More updates on will be posted on our study of microbial polysaccharide metabolising enzymes as it uncovers. |
| Group Members |
|
| Principal Investigator |
|
| Gopal R Periyannan | |
| |
|
| Graduate | |
| Hashni Epa Vidanage | |
| Undergraduate | |
| Current |
|
| Xa Burton |
|
| Andrew Hladilek | |
| Alumni |
|
| Graduate |
|
| Dilani G Gamage - PhD - Wayne State
University, Detroit, MI; Postdoctoral Fellow - Cicinnati Children's Hospital |
|
| Undergraduate |
|
| Andrew Bolokowicz |
|
| Brant Riegel | |
| Breanne Cornwell |
|
| Driscolle Augustine | |
| Jason DeGroate |
|
| John Saathoff | |
| Kevin Vessells | |
| Nicholas Kucinski |
|
| Timothy Russell | |
| Scott Keller | |
| Valencia Anderson |
| Publications |
|
1. Hu, Z.,
Periyannan, G.,
Bennett, B., and Crowder, M.W., “Role of the Zn1 and Zn2 sites
in metallo-β-lactamase L1” J. Am. Chem.
Soc. (2008) Oct 3 (Epub). 3. Amit Kumar, Gopal R. Periyannan, Beena Narayanan, Aaron W. Kittell, Jung-Ja Kim and Brian Bennett. Experimental evidence for a metallohydrolase mechanism in which the nucleophile is not delivered by a metal ion: EPR spectrokinetic and structural studies of aminopeptidase from Vibrio proteolyticus. Biochem J. (2007) May 1;403(3):527-36. 5.
A. L. Costello, G. R. Periyannan, K.-W. Yang, M. W. Crowder and D. L.
Tierney, "Site Selective Binding of Zn(II) to Metallo-b-Lactamase
L1 from Stenotrophomonas maltophilia", (2006) JBIC,
Apr;11(3):351-8.
6.
Gopal R. Periyannan, A.L. Costello, D.L. Tierney, K.W. Yang, B.
Bennett, and M.W. Crowder, "Sequential Binding of
Co(II) to Metallo-b-Lactamase
CcrA" Biochemistry (2006)
Jan 31;45(4):1313-1320. 7. G.P.K. Marasinghe, I.M. Sander, Brian Bennett, Gopal R Periyannan, Ke-Wu Yang, C.A. Makaroff, and M.W. Crowder, "Structural Studies on a Mitochondrial Glyoxalase II", J. Biol. Chem.(2005); 280 (49) 40668 - 40675. 8. Gopal R. Periyannan, Patrick Shaw, Tara Sigdel and Michael W. Crowder, “In vivo folding of recombinant metallo-b-lactamase L1 requires the presence of Zn(II)”, Protein Science (2004) 13: 2236- 2243.9. Anne L. Carenbauer,
James D. Garrity, Gopal R. Periyannan,
Robert B. Yates, and Michael W. Crowder, “Probing
substrate binding to Metallo-β-lactamase L1 from Stenotrophomonas
maltophilia by using site-directed mutagenesis”, BMC Biochem. (2002); 3
(1): 4. 10. Crowder,
Michael W., Yang, Ke-Wu, Carenbauer, Anne L., Periyannan, Gopal.,
Seifert, Mary E., “The Problem of Solvent Exposable
disulfide when Preparing Co (II)-Substituted Metallo-b-lactamase L1 from Stenotrophomonas
maltophilia”,(2001) JBIC 6, 91-99. |
| International
Proteolysis Society |
| International
Protease Network |
| MEROPS
- The Peptidase Database |
| ZINC
Database |
| International
EPR Society |
| National
Biomedical EPR Center |