Chemistry 1410
Spring 2005
Practice Problems – Chapter 14
![]()
1. Consider the reduction of carbon dioxide by hydrogen to give water vapor and carbon monoxide at 420oC.
H2
(g) +
CO2 (g) D H2O (g) + CO
(g) Kc
= 0.10
Suppose you have the following initial concentrations:
[H2]o = 2.50 M [CO2]o = 1.50 M [H2O]o = 2.00 M [CO]o = 1.00 M
Calculate
the equilibrium concentrations of all reactants and products.
Answer: [H2]eq = 3.09 M; [CO2]eq
= 2.09 M; [H2O]eq = 1.41 M;
[CO]eq = 0.41 M
2. Acetic acid (HC2H3O2) ionizes in water to form hydronium ion (H3O+ ) and acetate ion (C2H3O2- ) . The equilibrium can be represented below:
HC2H3O2
(aq) + H2O (l) D H3O+ (aq) + C2H3O2-
(aq) Kc = 1.8 x 10-5
What would be the equilibrium concentrations of acetic acid, hydronium ion, and acetate ion if the initial concentrations were:
[HC2H3O2]o = 0.00400 M [H3O+]o = 0.00 M [C2H3O2-]o = 0.00 M
NOTE: The chemical structures involved here are:
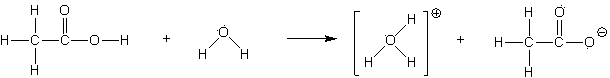
[HC2H3O2]eq = 0.00374 M [H3O+]o =
0.000259 M [C2H3O2-]o
= 0.000259 M