The parcel will find itself cooler than the environmental (sounding) temperature.
At the same pressure, a cooler parcel will be more dense than the environment.
Being denser, the parcel will descend back to where it came from.
STABLE!
To make a cloud we need:
Definitions
Adiabatic - A process in which heat is neither
added nor subtracted from the system.
Diabatic - A process in which heat is added
or
subtracted from the system, e.g., solar heating, radiation cooling.
For example:
When the bicyclist runs over the nail, the air, having
a higher pressure than the outside air, will rush out. The air
does
work against the atmosphere as it rushes out from the tire. In
doing
this work of displacing the outside air, the air from the tire must use
some energy. That energy comes from the kinetic molecular
energy.
The kinetic energy of the molecules from the tire slows and the
temperature
falls.
No heat has been added or removed from the system
yet
the expanding air cools.
This process is called Adiabatic Cooling.
Also called Expansional Cooling.
This process is reversible.
If we took a pump to compress the air, as we would
if
we were filling the tire, then the energy used to compress the air is
used
to increase the kinetic energy of the molecules. Compression
warms
the air.
This process is called Adiabatic Warming.
For example, if we raise a parcel of air from ground level to 100 meters in height, the temperature will decrease by 1°C. The parcel cools at a rate of 1°C per 100 m or 10°C per km.
The parcel expanded and did work on its environment!
Now, bring the parcel back down to the surface. The environment did work on the parcel.
This is an adiabatic process and is reversible.
Example 2: If we use a moist parcel of air (RH = 100%)
The rising air cooled and produced condensation. The condensation released latent heat so the rising parcel does not cool as rapidly with height as a dry parcel.
Parcel cools only 0.6°C per 100 m (on average).
Moist adiabatic lapse rate = 0.6°C per 100 m.
Remember -- This is an average lapse rate. The
actual one varies!!!
If the moisture falls out of the parcel as rain, the
process is not reversible.
Reversible only if no moisture has been removed!
If we (somehow) lift the parcel: It will cool at the
dry
adiabatic lapse rate.
The parcel will find itself cooler than the
environmental
(sounding) temperature.
At the same pressure, a cooler parcel will be more
dense
than the environment.
Being denser, the parcel will descend back to where it
came from.
STABLE!
If we (somehow) lift the parcel: It will cool
at
the dry adiabatic lapse rate.
The parcel will find itself warmer than the
environmental
(sounding) temperature.
At the same pressure, a warmer parcel will be less
dense
than the environment.
Being less dense, the parcel will ascend and move
farther
from where it came from.
UNSTABLE!
If we (somehow) lift the parcel: It will cool
at
the dry adiabatic lapse rate.
The parcel will find itself at the same temperature
than
the environmental (sounding) temperature.
Being the same density, the parcel will not be
accelerated
in any direction and will remain where it is.
NEUTRAL STABILITY! -- Dry Neutral, or Conditional
Instability
So ...
We can evaluate the stability of an atmospheric layer
by comparing the sounding to the dry and moist adiabats.
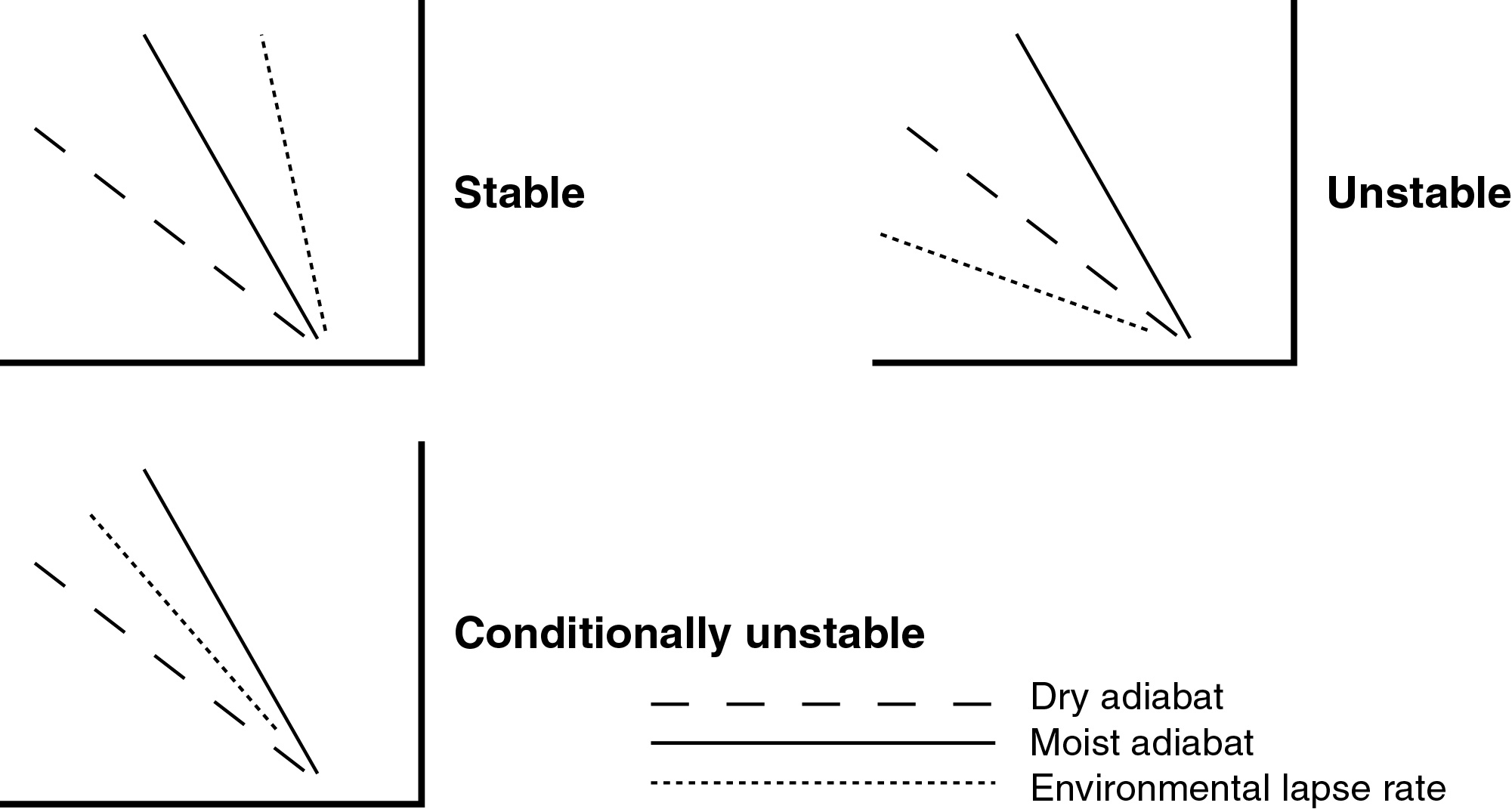
Things to realize from these diagrams:
Now all we have to do is get the parcel of air
lifted.
We can do that in four ways:
Orographic Lifting
Adiabatic Warming (Leeward Side)
Adiabatic Cooling
(Windward Side)
Therefore it is usually wetter on the windward
side than
on the leeward side.
Surface Boundaries
Convection
Cloud Formation
When we lift the air, where will condensation occur?
Depends on the moisture content of the air that is being lifted.The lifting condensation level (LCL) is the altitude, usually expressed as a pressure, at which the lifted air is cooled dry adiabatically to saturation.
Moist air requires less cooling, hence less lifting, to reach the dew point.
Drier air requires more cooling and more lift to reach the dew point.
Clouds will form at this level.
As an air parcel rises and cools, the saturation
mixing
ratio decreases.
The actual mixing ratio does not change.
When the parcel cools to the point when the parcel
mixing
ratio and the saturation mixing ratio are equal, RH will be 100% and a
cloud will form.
If lifting continues, the parcel will rise moist adiabatically (making a cloud).
Clouds are a visible manifestation of condensation or deposition in the atmosphere.
How can chance collisions of water vapor molecules lead to the formation of cloud droplets that will be long-lived?
If more water is added such that the atmosphere is supersaturated (RH ~300 %), then water molecules can form a stable droplet. This process is called homogeneous nucleation.
We can measure the amount of moisture in the air and find that the cloud droplets form when the air just reaches saturation. Why?
Recall that dew and frost form on grass or other things. The water vapor molecules need a "gathering place".
Nearly a century ago it was discovered that the atmosphere contains particles that have an affinity for water. These serve as centers for condensation. They are called Cloud Condensation Nuclei (CCN).
With CCN, we need much smaller supersaturations (RH >100%). In nature we find supersaturations on the order of 1.5%.
The atmosphere has plenty of CCN:
The formation of cloud droplets using CCN is called: heterogeneous nucleation.