

We see some objects--like the stars or our Sun or a candle flame--because of light that comes directly from them; that light enters our eyes and we see the object.
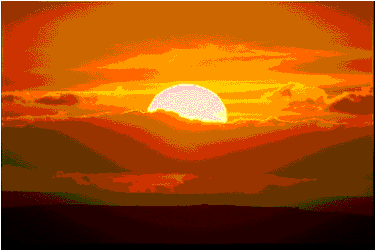




We see most other objects because light that originates from some other source strikes these objects and then enters our eyes and we see the objects.


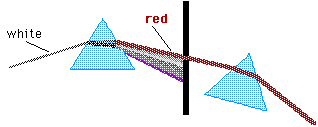
Unless or until we make extremely careful observations, it seems clear that light travels in straight lines. We can hear a band around the corner of a building but we can not see it. Sound waves bend around obstacles but light does not seem to in common situations. All of our surveying--or something as common as reaching for a doorknob--relies on the idea that light travels in straight lines.
Sir Isaac Newton described light as tiny particles which he called corpuscles. This particle nature of light is consistent with light traveling in straight lines.
Christian Huygens, a contemporary of Newton's, thought that light was a wave.
In 1801, the British scientist Thomas Young reported experiments on the interference of light passing through a double slit which unequivocally proved light had to be a wave.
 In 1905, Albert Einstein explained details of the
photoelectric effect and was later awarded the Nobel
Prize for this work (not for his work on Relativity!). To explain the
photoelectric effect, Einstein required that light be a collection of
particles or lumps. We can call these lumps of light
"photons" or "quanta".
In 1905, Albert Einstein explained details of the
photoelectric effect and was later awarded the Nobel
Prize for this work (not for his work on Relativity!). To explain the
photoelectric effect, Einstein required that light be a collection of
particles or lumps. We can call these lumps of light
"photons" or "quanta".
Curiosier and curiosier, eh?
Today it is difficult to recall or even to imagine how very controversial Einstein's Theory of Relativity was in the first half of the twentieth century. Clearly, Einstein is today remembered for his Theory of Relativity. But at the time, it was extremely controversial. His explanation of the photoelectric effect was not controversial.
Is light a wave? Young's double slit experiment seems to clearly prove that light is a wave. Is light a particle? The photoelectric effect seems to clearly prove that light is a particle. Waves and particles seem to be contradictory. Everything I know or use to describe a wave is inconsistent with my knowledge and description of a particle. And vice versa. Not only does light present us with this delima, but electrons ant atomic theory present the same problems. Are electrons waves? Are electrons particles. There is firm experimental evidence for both. This problem occupied the best and brightest minds in Physics for the first several decades of the previous century.
Finally, Niels Bohr, from the University of Copenhagen, described this as the "Complimentarity Principle". In Bohr's view, the wave model and the particle model should be considered as complimenting each other, rather than contradicting each other. The "real" nature of light and electrons is more complex than either our wave model or our particle model. Both models are necessary to understand the nature of light or electrons -- both models compliment each other! This is also referred to as the "wave - particle duality of Nature".

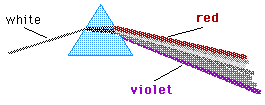
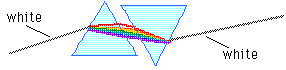
(C) 2003, Doug Davis; all rights reserved