

Ch 19, Temperature Thermal Expansion
What happens to the length of a rod when you heat it?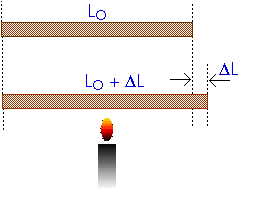
In a Physics experiment, a bar of aluminum, initially 80 cm (0.80 m) long, is brought from room temperature of 23oC to 100oC by surrounding it with steam. By how much does the aluminum bar expand?
From a table we find that the coefficient for linear expansion for aluminum is
= 24 x 10 - 6 (Co) - 1
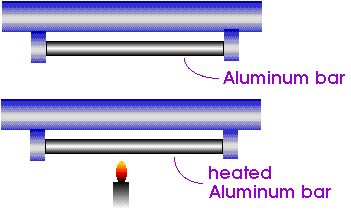
describes what happens when a rod is heated. For such a rod, we would only be interested in its length.
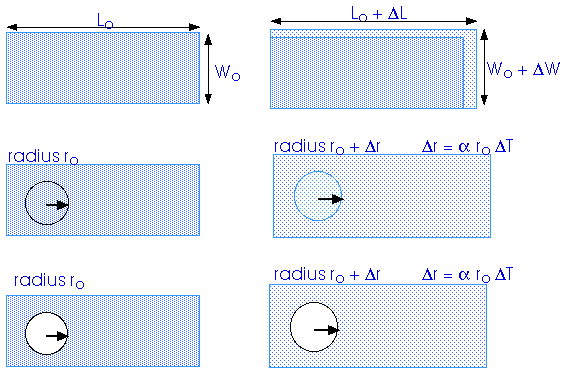
What happens when a plate is heated? And what happens to a hole cut into a plate as the whole thing is heated? If you cut a hole out of a piece of dough and bake it -- as in a donut -- the dough will expand and make the hole smaller. That is not the case for heating a metal plate. The hole expands along with the rest of the plate.
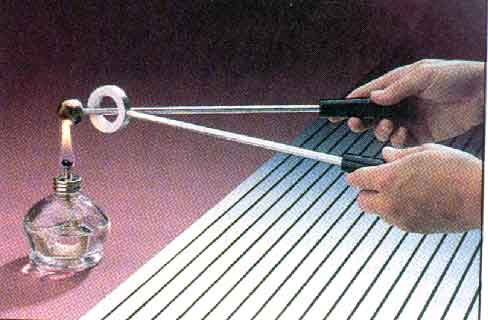
This can be very useful in a bimetallic strip -- made of two different materials with different coefficients of thermal expansion.
A bimetallic strip can be used as a thermometer. It can also be used in a temperature-sensitive switch.
Bimetallic strips are used in most ordinary household thermostats. To get a larger deflection, such a bimetallic strip may be fashioned into a coil so that it takes up little space.
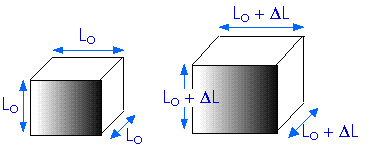
What about a three-dimensional volume? How does heating affect that?
Common, ordinary, necessary water is an exception. Like other liquids, water contracts as it gets colder -- until it reaches 4oC. Then it expands! Think of what this means to the density. As water gets colder, its density increases until it reaches 4oC. Then its density decreases. As water gets colder, its density increases -- meaning it will drift to the bottom of, say, a lake -- until it reaches 4oC. Then its density decreases -- causing it to float to the top of, say, a lake.
If water behaved as other liquids, as the air temperature became colder, the surface water would become colder and would drift to the bottom. The temperature of lakes would be fairly uniform and lakes would freeze from the bottom up -- and, thus, would freeze solid. This would mean that fish could not survive winters outside the tropics.
However, water behaves very differently compared to other liquids. As the air temperature becomes colder, the surface water becomes colder and drifts to the bottom. Until the surface water temperature reaches 4oC. Then additional cooling of the water makes that colder water more bouyant and it remains at the surface. Therefore, freezing occurs at the top of a lake and the water underneath remains liquid -- allowing fish to survive the winter!
Thermal Stress
Return to Temperature ToC (c) Doug Davis, 2002; all rights reserved