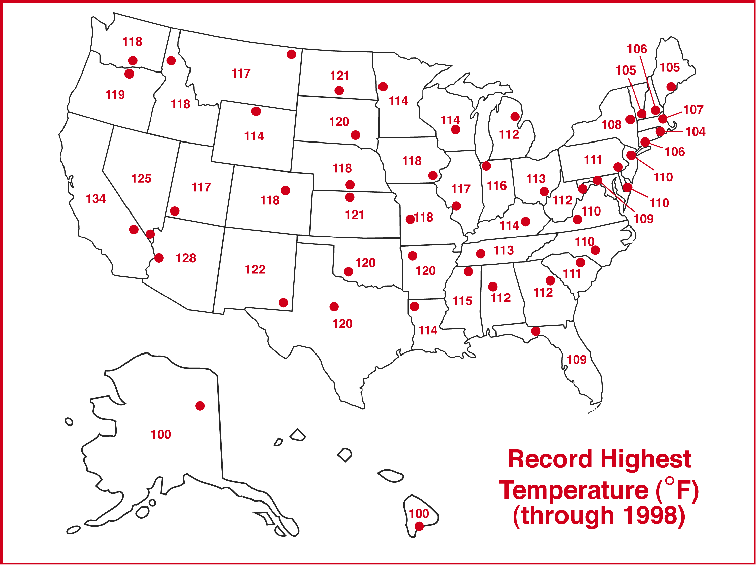
(From http://www.ncdc.noaa.gov/ol/climate/severeweather/temperatures.html)
Temperature - a measure of the average kinetic energy of the molecules in that substance. Therefore, when we talk about the temperature of the air, we're actually talking about how fast the air molecules are moving.
Temperature (K.E.) ~ mv2
Therefore as temperature increases the velocity of the air molecules increases.
Extremes of recorded temperature
Highest temperature: +136°F (57.8°C) at El Azizia,
Libya
Lowest temperature: -126°F (-87.8°C) at
Vostok Station, Antarctica
US highest temperature: +134°F (56.6°C) at Death
Valley, California
US lowest temperature: -80°F
(62.2°C) at Prospect Creek, Alaska
IL highest temperature: +117°F (47.2°C) at East
St. Louis (1954)
IL lowest temperature: -36°F
(-37.8°C) at Congerville (last January)

(From http://www.ncdc.noaa.gov/ol/climate/severeweather/temperatures.html)
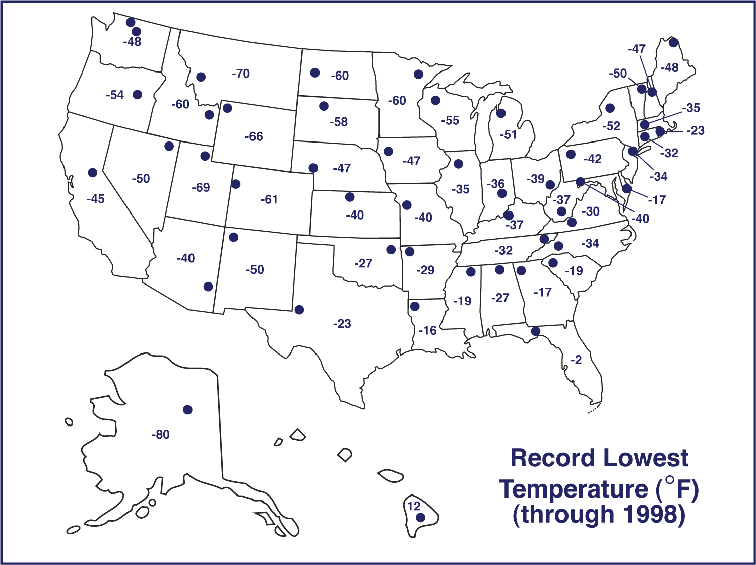
(From http://www.ncdc.noaa.gov/ol/climate/severeweather/temperatures.html)
Temperature Scales
In this course you should (will) become familiar with
three temperature scales and their units:
Fahrenheit (°F) -- German
Celsius (°C) -- Swedish
Absolute (K) -- "Scientific"
Fahrenheit Scale
Fahrenheit Scale (1714):
Ice melts at 32°F,
Water boils at 212°F.
N.B.: 180 degrees between melting and boiling point of
pure water at sea level.
Celsius Scale
Celsius Scale (1742):
Ice melts at 0°C
Water boils at 100°C
N.B.: This scale was originally upside down. It
is one of several "Centigrade Scales." 100 degrees between melting
and boiling point of pure water at sea level.
Thermodynamic (Kelvin) Scale
Kelvin or Absolute Scale (1800's):
No molecular motion at 0 K; use Celsiusí degree
increment
Ice melts at 273 K
Water boils at 373 K
First, a little about radiation. It can be described by its wavelength and frequency.
Wavelength - the actual length, in meters, between any two crests (or troughs) in the wave, e.g., radio waves have a wavelength from ~100cm to 100s of meters; light has a wavelength of ~10-9 m. Shorter wavelength radiation would be x-rays, UV rays, and light; longer wavelength radiation might be infrared, radar, TV, and radio.
Frequency - the number of wave crests that pass a particular point every second, measured in Hertz (Hz).
The relationship between wavelength and frequency is simply:
c = ln
c = speed of light (3 x 108 ms-1)
l = wavelength in meters,
m
n = frequency in Hertz, Hz
All things radiate!
Two laws describe how all things radiate:
1) Amount of energy they radiate (Stefan-Boltzman Law)
2) Maximum energy at which they radiate (Wein's Law)
Stefan-Boltzman Law: E = sT4
E - amount of energy being radiated (Wm-2)
s - Stefan-Boltzman constant, 5.67 x 10-8 Wm-2K-4
T - temperature, K
Wein's Law: the wavelength of radiation that
an object radiates in inversely proportionate to its temperature, i.e.,
hotter objects radiate at shorter wavelengths.
lmax = (2897 mm K) (T-1)
E.g., where does the Sun emit most of its radiation?
T = 6000 K
lmax = (2897 mm
K) / (6000 K) = 0.48 mm (green light)
"Composition" of Solar Radiation
7 % Ultraviolet and shorter
X-rays, gamma rays, etc.; these are higher energy waves44 % Visible light - what we can see with our eyes - we can see from approximately 0.4 to 0.7 mm
Earth only receives ~ one two-billionth of the Sun's energy, but this amount accounts for 99.9 percent of the energy that the Earth uses to heat the planet (remaining from internal sources).
Heat is a form of energy transfer from one place to another due to differences in temperature.
Things donít have "heat" but they can be heated.
Energy
Energy is the ability or capacity to do work on some
form of matter.
There are several forms of energy, including the following:
The First Law of Thermodynamics states that
energy lost during one process must equal the energy gained during another.
Energy is neither created nor destroyed.
Methods of Energy Transfer
As the Earth orbits the Sun, there are three orbital factors that effect the amount of energy the Earth receives:
Since the Earth is tilted in it's orbit, not all the Earth
receives the same amount of energy.
(Figure 2-2, page 29 in Lutgens and Tarbuck's The Atmosphere,
2001) Schematic showing reduced effectiveness of the Sun to heat as
the angle at which the rays pass through the atmosphere decrease (hence
they must pass through more of the atmosphere)
More energy is received at the equator than at the poles owing to the obliquity of the Earth's axis.
Solstices and Equinoxes
Since the Earth is tilted 23.5° on its axis, there
is only one latitude on the Earth at any one time when the Sun's rays are
striking at 90° (Sun is directly overhead).
As the Earth moves in its orbit, that latitude changes
from a maximum of 23.5°S latitude to 23.5°N latitude. Theses
latitudes are given the special names of the Tropic of Capricorn (23.5°S)
and Tropic of Cancer (23.5°N) [note typo in book, p.31 in figure]
(Figure 2-4, page 30 in Lutgens and Tarbuck's The Atmosphere,
2001) Schematic showing solstices and equinoxes.
Reflection, refraction, and albedo
Okay, so now we know that the Earth gets most of its energy from the Sun. However, much of the energy that gets to Earth's upper atmosphere never makes it to the surface.
Radiation can be absorbed, reflected, scattered, refracted, or transmitted.