Welcome!!!
(page under construction)
The Treadwell group is interested in several areas of organic chemistry, each of which will be described below. Please feel free to contact me with any questions or comments you may have.

| Methodology | Natural Products | |
|---|---|---|
| Past Students | Publications |
|
Area 1: Sequential Condensation and Annulation Methodology Employing 1,3-Bisphosphonoacetone
One of the challenges of organic chemistry is to synthesize large or complicated molecules efficiently and with good control over issues of selectivity. While there are already many reactions and approaches that can be found in literature, there is always the search to do something better or differently..
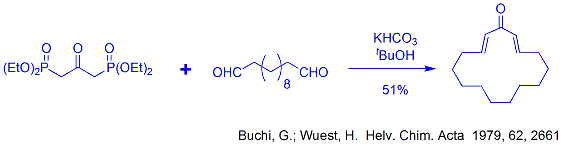
Our methodology centers around the use of 1,3-bisphosphonoacetone to make unsymmetrical alkenes and hydrindanones. 1,3-Bisphosphonoacetone was first used by Buchi et al to make perfumery compounds, by reacting it with linear dialdehydes to form a macrocyclic dienone.
So this worked effectively reacting BOTH ends of the bisphosphonate in the reaction, but we were curious if there was more applicability to this reagent? Could we react only ONE end of the compound in one reaction, then go and use the OTHER end in a later step (as shown below)?
Well the answer to this was YES, so we used about 15 different aldehydes in the first step, to test the scope and applicability, and then about 10 different aldehydes in the second step. But this was only the beginning of the project...
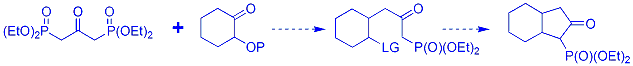
..Because then we wondered if we could use BPA (bisphosphonoacetone) to quickly build polycyclic systems? This would involve doing a monocondensation reaction with a cyclohexanone that had a leaving group motif alpha to the carbonyl.. After the condensation reaction, the C=C bond could be reduced away, and the ring closed by removing the acidic alpha hydrogen to the phosphonate (as shown below)..
Another area that we are currently working on involves making organic compounds that have useful properties that could be used in the design and use of new materials. In particular we are interested in a particular property called photochromism - compounds that exhibit this property reversibly change their color when taken from the dark to the light or vice versa. And more specifically, we are looking at a compound that is photochromic in the solid state - so if you have a vial of the crystals they will display this remarkable transformation (many photochromic compounds only display photochromicity when in dissolved in solution)..
The compounds themselves are rather easy to make (one step if the right starting material can be bought, about seven steps if not), and are nicely purified by recrystallization. We explore these compounds by the usual spectral techniques (NMR, IR, and UV-VIS spectroscopy) as well as X-ray diffractometry, and hope to elucidate an understand of how the photochromicity can be tuned by varying the substituents in the structure. We would also like to add a computational chemistry component into this research project, to help direct and understand the observed behaviors.
Area 3: Natural Products / Chemical Ecology
Natural products chemistry focuses on isolating and identifying secondary metabolites from natural sources, be it plant, animal, or microbial. Secondary metabolites are compounds produced by an organism that are not directly involved in the basic life function of the organism (so for instance DNA and sugars are primary metabolites, while nicotine and coniine are secondary metabolite). Chemical ecology is focused on realizing how these secondary metabolites govern the interactions of different speices (for instance, citronnel repels insects, hence decreasing herbivory on the plant). These two disciplines can work powerful together, in that a particular behavior can be identified in the wild, and then the chemical responsible for this behavior isolated in the lab, and assays with the isolated compound used to confirm that it is the active agent.
There are currently 2 projects our group is investigating in this area.. These projects rely extensively on NMR spectroscopy, and one of the particular challenges encountered is simply the limited amount of starting materials - when we run out something, it can't be reordered, but needs to be reisolated...
A. Isolation and Elucidation of Birch metabolites

USDA-NRCS PLANTS Database / Herman, D.E., et al. 1996. North Dakota tree handbook. USDA NRCS ND State Soil Conservation Committee; NDSU Extension and Western Area Power Administration, Bismarck. One ongoing project is simply to investigate the secondary chemistry of other birch species that haven't been looked at before. This involves preparation of the plant sample (debudding and cutting to appropiate size), steeping the material in an organic solvent, removal of the solvent, and then flash chromatography to separate and isolate individual compounds. The structure of any new compounds is determined primarily using NMR spectroscopy - 1H and 13C NMR, DEPT, COSY, HMBC/HSQC, and NOESY spectra. [DEPT tells you how many hydrogens are on each carbon, HMBC allows you to tell which hydrogens are on which carbon, HSQC tells you which carbons are close to a given hydrogen, COSY tells you which hydrogens are coupled to each other, and NOESY tells you which hydrogens are close in space to each other]. It's like trying to solve a very complicated jig-saw puzzle to 100% confidentally determine the structure.
A new project that will be commencing soon is a collaborative one that will seek to identify on a biochemical and molecular biological level how one of the birch metabolites acts a feed deterrent.
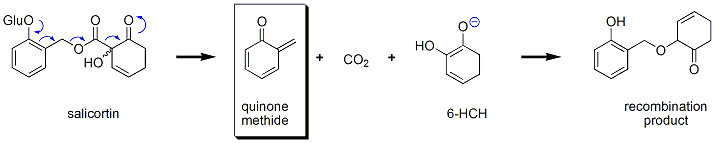
B. Determination of the absolute stereochemistry of salicortin
© 1995 Saint Mary's College of California
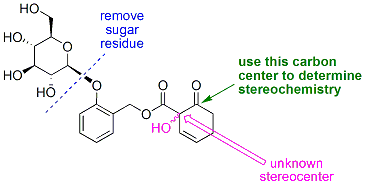
There is however one remaining question regarding salicortin - what is the absolute stereochemistry at the remote quaternary carbon? While this sounds trivial on paper, determing it experimentally is a greater challenge, one that we seek to answer by chemical degradation. What we will do is modify the structure of the natural product a slight bit, then break it apart (to "get rid of" the five other stereocenters located in the sugar residue), and use NMR methods (Mosher methodology) to determine the absolute stereochemistry.
Former students (the ones who really did the work, who I'm very thankful and proud of) in the group and what they're up to now..
James Kelsheimer (CHEM)- graduated Dec. 2002. Now works in Indiana helping to destroy army chemical munitions.
Casey Carnes (BIO) - graduated May 2003.
David Zigler (CHEM) - graduated Dec. 2003. Earned PhD in Chemistry from Virginia Tech, postdoc'ing in California
Melissa Tonsor (BIO) - graduated May 2004.
Bret Van Ausdall (CHEM) - graduated May 2005. Working on PhD in Chemistry at University of Utah.
Kelly Cosgrove (CHEM) - graduated May 2006.
Neely Diers (FCS) - graduated May 2004. Earned MS in Food Chemistry from Southern Illinois.
Brendan Aydt (CHEM) - graduated Dec. 2005. Teaches HS science in Illinois.
Brett Hurlimann (BIO) - graduated May 2005. Finished MD in Rockford Illinois, currently resident in pediatrics.
Reto Frie (CHEM) - graduated May 2006. Working on PhD in Chemistry at University of Wisconsin.
Russell Tett (BIO) - graduated May 2006.
Tom Wysocki (HIST) - graduated May 2007.
Phil Brewer (BIO) - graduated May 2006.
Josh Bland (BIO) - graduated May 2006. Working on degree in Biomedical Engineering at University of Chicago.
George Hudson (CHM) - graduated May 2007. Working on PhD in Chemistry at Southern Illinois.
Ryan Summers (BIO) - graduated May 2007.
Chris Szczesniak (BIO) -
Kenzi Murray (BIO) - graduated May 2008. Working for Marathon Oil.
Opal Bacon (BIO) -
Mark Heywood (BUS) - graduated May 2009.
(Underline indicates undergraduate contributor, italics and underline indicates graduate contributor).
- Bland, J. B.; Davis, G. S.; Treadwell, E. M. Determination of the Absolute Stereochemistry of (+)-Neomenthol using Mosher Esters. J. Undergrad. Chem. Res., 2010, 9, 32-35.
- Treadwell, E. M.; Clausen, T.P. Is Hedysarum Mackenzii (Wild Sweet Pea) Actually Toxic? Ethnobot. Res. Appl.2008, 6, 319-320.
- Treadwell, E.M.; Lin, T.-Y. A More Challenging Interpretative Nitration Experiment Employing Substituted Benzoic Acids and Acetanilides. J. Chem. Ed. 2008, 11, 1541-1543.
- Treadwell, E. M. 4'-Methylchalcone. Acta Crystallographica Section E 2006, E62, o5899-o5900.
- Treadwell, E. M.; Black, T. H. An Engaging Illustration of the Physical Differences among Menthol Stereoisomers. J. Chem. Ed. 2005, 82, 1046-1048.
- Reiter, E.; Treadwell, E.; Cederstrom, E.; Reichardt, P. B.; Clausen, T. P. Diterpenes from Colophospermum mopane: “Missing Links” in the Biogenesis of 9,13-Epoxylabdanes. J. Nat. Prod. 2003, 66, 30-33.
- Treadwell, E. M.; Neighbors, J. D.; Wiemer, D. F. A Cascade Cyclization Approach to Schweinfurthin B. Org. Lett. 2002, 4, 3639-3642.
- Treadwell, E. M.; Cermak, S. C.; Wiemer, D. F. Synthesis of Schweinfurthin C, a Geranylated Stilbene from Macaranga schweinfurthii. J. Org. Chem. 1999, 64, 8718-8723.
- Green, T. P.; Treadwell, E. M.; Wiemer, D. F. Arieianal, a Prenylated Benzoic Acid from Piper arieianium. J. Nat. Prod. 1999, 62, 367-368.
- Zhu, J. J.; Withers, S. G.; Reichardt, P. B.; Treadwell, E.; Clausen, T. P. Salicortin: a Repeat-Attack New Mechanism-Based Agrobacterium faecalis -Glucosidase Inhibitor. Biochem. J. 1998, 332, 367-371.
Posters
(Underline indicates undergraduate contributor, italics and underline indicates graduate contributor).
- Fortin, S. M.*; Murray, K. M.; Wysocki, T. J.; Treadwell, E. M. Synthesis of Cinnamaldehyde Semicarbazones to Explore their Photochromicity (Poster 20). East Central Illinois ACS Section, Women’s Chemist Committee Undergraduate Research Symposium, April 24, 2010. Champaign, IL.
- Fortin, S. M.*; Murray, K. M.; Wysocki, T. J.; Treadwell, E. M. Synthesis of Cinnamaldehyde Semicarbazones to Explore their Photochromicity (CHEM 16). 102nd Annual Meeting of the Illinois State Academy of Science, April 9-10, 2010. Decatur, IL.
- Hudson, G. A.*; Van Ausdall, B. R.; Keiter, R. L.; Treadwell, E. M. Pursuing 3,3-diphenyl-1,2-bis(diphenylphosphino)propane and 3,3-diphenyl-1,2-bis(diphenylphosphino)butane (CHED 649). 233rd National Meeting of the American Chemical Society, March 25-29, 2007. Chicago, IL.
- Breen, M. E. *; Cagle, K. S.; Cuddy, M. F.; Grove, R. C.*; Schwenk, D. S.; Unterfenger, M.; Treadwell, E. M.; Tremain, S. M. Student Affiliates Activities at Eastern Illinois University (CHED 1558). 233rd National Meeting of the American Chemical Society, March 25-29, 2007. Chicago, IL.
- Bland, J. B.*; Treadwell, E. M. Studies towards Absolute Stereochemistry Determination of Salicortin and Tremulacin. (ORGN 159) 41st Annual Midwest Regional Meeting of the American Chemical Society, October 25-27, 2006. Quincy, IL.
- Zigler, D. F.; Hudson, G. A.; Aydt, B. P.; Cosgrove, K. C.; Van Ausdall, B.; Treadwell, E. M.* Mono- and sequential condensations of 1,3-Bis(diethylphosphono)acetone (ORGN 493). 232nd National Meeting of the American Chemical Society, September 10-14, 2006. San Francisco, CA.
- Treadwell, E. M. A More Challenging Aromatic Nitration Experiment. (CHED 224) 37th Annual Great Lakes Regional Meeting of the American Chemical Society, May 30-June 2, 2006. Milwaukee, WI.
- Van Ausdall, B.*; Keiter, R.L.; Treadwell, E.M.; Keiter, E.A. The Synthesis of 1,2-bis-(Diphenyl-phosphino)-1-Diphenylpropane. 19th National Conference on Undergraduate Research, April 21-23, 2005. Lexington, VA.
- Cosgrove, K. A.*; Zigler, D. F.; Van Ausdall, B.; Kelsheimer, J. W.; Aydt, B. P.*; Treadwell, E. M. Sequential Condensations of 1,3-bis(diethylphosphono)acetone. (CHED 244) 36th Annual Great Lakes Regional Meeting of the American Chemical Society, October 17-20, 2004. Peoria, IL.
- Van Ausdall, B.*; Zigler, D. F. ; Treadwell, E. M. Sequential Condensations of 1,3-Bisphosphono-
acetone. 16th Annual Butler University Undergraduate Research Conference, April 2,
2004. Indianapolis, IN.- Van Ausdall, B.*; Treadwell, E. M. Monocondensation Reactions Involving 1,3-bis(diethyl-phosphono)acetone. 9th Annual EIU College of Sciences Science Fest, March 26, 2004. Charleston,IL.
Oral
- Treadwell, E. M.*; Wiemer, D. F. Progress Toward the Synthesis of Schweinfurthins A and B. 220th ACS National Meeting, August 20-24, 2000, Washington, D.C.
- Treadwell, E. M.*; Cermak, S. C.; Blunt, S. B.; Wiemer, D. F. Towards the Syntheses of the Schwein-furthins. 33rd Annual Midwest Regional ACS Meeting, November 4-7, 1998,
- Clausen, T. P.*; Reiter, E. M.; Treadwell, E.; Zhu, J. J.; Martin, M. A New Irreversible Inhibitor of Almond -Glucosidase with an Unusual Repeat Attack Mechanism of Deactivation. 36th Annual ASP Meeting, July 23-27, 1995, Oxford, MS. Wichita, KS.