
PHY 1361
First Hour Exam
February 7, 2001

PHY 1361
First Hour Exam
February 7, 2001
Statistics:
High: 98%Mean: 75%
Low: 55%
| Return to PHY 1361 Calendar |
![]()
Pace yourself! Do not spend too much
time on any one particular problem or question. There are four
calculational problems and four short-essay, quickie
discussion questions.
Read through the entire exam first. If you find one of the questions
or problems is taking too long, leave it and go on to others.
You can come back to that problem later if you have time.
Problem 3 is clearly the longest and most involved; allot a little
more time for Problem 3.
Entropy is important. In picking and choosing topics for questions, I
did not choose an entropy question for this hour exam. I expect ideas
of entropy to reappear on the final exam.
1. (19.10) The concrete sections of a certain
superhighway are designed to have a length of 25.0 m. The sections
are poured and cured at 10.0oC. What minimum spacing
should the engineer leave between the sections to eliminate buckling
if the concrete is to reach a temperature of 50.0oC?
How much will the 25.0 m section expand as the temperature changes
from 10.0oC to 50.0oC?
For concrete, the coefficient of thermal expansion is
The temperature change, ![]() T,
is 40 Co;
T,
is 40 Co; ![]() T
= 40 Co
T
= 40 Co
Now we can put all the pieces together and evaluate the expansion,
![]() L
;
L
;
Since each concrete section will expand by 1.2 cm,
there must be 1.2 cm between each section so they do not run into
each other and cause compressive stresses.
2. (similiar to 20.8) A calorimeter contains 500 mL of water
at 30oC and 300 g of ice at 0oC.
If all the ice melts, determine the final
temperature of the system.
If the final temperature is 0oC, determine how much ice
melts.
Now, how much heat is gained by the ice? Does all
of the ice melt? We do not know. So we must make an assumption -- or
a guess. We then have to see if our result is consistent with that
assumption.
Assumption: All the ice melts and Tf >
0oC
Be sure to check for consistency with our final answer and this
assumption.
This ice is initially at 0oC so heat that it gains
immediatly starts to melt the ice. Remember, mice =
m2 = 300 g = 0.300 kg. m2 is the mass of the
ice after it has melted and is water at 0oC.
Is that "believeable" or "reasonable"? It is
between the lowest and highest initial temperature so that means it
is, at least, "possible". No, this is colder than the ice! Our
assumption was wrong! We must make another
assumption.
Assumption: Tf = 0 and only part of the ice
melts. Now we must determine how much of the ice
melts.
This ice is initially at 0oC so heat that it gains immediatly starts to melt the ice. Remember, this time when some of the ice melts it does not change its temperature since Tf = 0oC. We are now looking for Mmelt .
As always, ask if this is "believeable" or
"reasonable" -- or consistent with our assumptions. Yes, this
is consistent with our assumption of only part of the ice
melting. This amount of ice that melts is, indeed, less than
our total, initial amount of ice. So this answer seems
plausible.
3. (similar to 21.26) Four liters of a monatomic
ideal gas confined to a cylinder are put through a closed cycle. The
gas is initially at 1.0 atm and at 300 K.
First, its pressure is tripled under constant volume.
It then expands isothermally to its original pressure and
finally is compressed isobarically to its original volume.
(a) Draw a PV diagram of this cycle.
Here is a sketch:
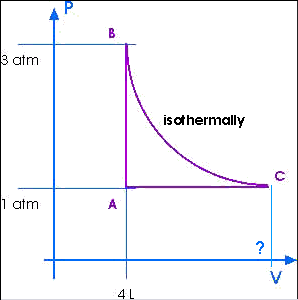
(b) What is the temperature of the isotherm?
(c) What is the volume at the end of this isothermal expansion?
VC = 12 L
(d) How much work is done in each process of this cycle?
(e) What is the net work done during this cycle?
4. (22.7) An engine absorbs 1 600 J from a
hot reservoir and expels 1 000 J to a cold reservoir in each
cycle.
(a) What is the efficiency of the engine?
e = 1 - [ 1 000 J/1 600 J]
e = 1 - 0.625
e = 0.375
e = 37.5%
(b) What is the power output of the engine if each
cycle lasts for 0.30 s?
For each cycle,
Then the power is
P = 2 000 W = 2 kW
Conceptual Questions --
A. (19.Q14) Metal lids on glass jars can
often be loosened by running them under hot water. How is this
possible?
The coefficient of thermal expansion is much greater for metals than for glass. Heating a metal lid on a glass jar will cause the metal lid to expand far more than the glass. This should allow you to unscrew the metal lid from the glass jar.
B. (20 “Opener") Why can you get
"burned" by biting into the hot CHEESE on a steaming pizza far easier
than by biting into the CRUST of the pizza?
Cheese is primarily water with a large heat capacity while the crust has very little water and a very small heat capacity.
C. (Q21.2) One container is filled with helium gas and another
with argon gas. If both containers are at the same temperature, which
molecules have the higher rms speed?
At the same temperature, the average value of the translational kinetic energy [(1/2) m <v2>] will be the same for both helium and argon. Argon has a larger mass so it will have a smaller value of <v2> or vrms. Helium has a smaller mass so it will have a larger value of <v2> or vrms.
D. (Q22.3) Use the first law of thermodynamics to explain why the
total energy of an isolated system is always constant.
The first law of thermodynamics is just a restatement of Energy Conservation. If a system is isolated no work is done on it and no heat is transferred to or from it. Energy may be transferred from one part of the system to another but the total energy remains constant.
| Return to PHY 1361 Calendar |
![]()
(C) 2001; Doug Davis; all rights reserved