

Kinetic Theory of Gases
Distribution of Molecular Speeds
NV, the Maxwell-Boltzman distribution function, describes the distribution of molecular speeds observed in a gas at some temperature T. N is the total number of molecules. Nv dv is the number of molecules found to have speeds between v and v + dv. We can also express these ideas in terms of probability. Nv dv/N is the probability of finding a molecule with speed between v and v + dv.
This Maxwell-Boltzman distribution function is given by
With this description of the speed distribution, we also find the following values:
vrms is the "root mean square" value of the speed,
<v> is the average value or the mean value of the speed (of course, the mean value of the velocity is zero!),
And vmp is the most probable value. The speed distribution curve has a maximum for v = vmp,
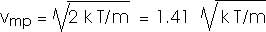
This graph shows the Maxwell-Boltzman distribution function for two different temperatures.
Return to Ch21 ToC (c) Doug Davis, 2002; all rights reserved