

Ch 19, Temperature Temperature and
the Zeroeth Law of Thermodynamics
Temperature is a description of how hot or cold something is.
Temperature can be measured by observind a change is some thermometric property of an object.
Simply touching something is one way to tell whether it is hot or cold or how warm it is. However, our sense of touch is not very accurate and can be fooled. If you reach into a freezer and pick up a metal ice-cube tray and a box of film you have stored there the ice-cube tray will feel colder than the film box--even though they are at the same temperature. The metal ice-cube tray is a good conductor of heat while the cardboard film box (and the plastic film canister inside) is a poor conductor of heat. Soak your left hand in a bucket of warm water and soak your right hand in a bucket of cold water. Now reach for a can of soda. To your left hand the can will feel cooler and to your right hand it will feel warmer. We need something more objective -- and more quantitative -- than our sense of touch.
Measurable properties of materials change with temperature; these are known as thermometric properties. The volumes of most liquids increase with temperature. The length of a metal rod increases as the temperature increases. The pressure of a constant volume of gas increases with temperature. The volume of a gas at constant pressure -- like the balloon in our earlier example -- increases with temperature. The electrical resistance of a piece of wire changes with temperature. Any or all of these may be used to define a temperature. Such properties are known as thermometric properties.
The thermometer you most readily think of is probably a liquid-in-glass thermometer like the one sketched here. Mercury or colored alcohol is contained in a bulb at the base of a thin tube of glass. The change in the length of the glass tube with variation in temperature is so small that we can ignore that and concentrate on the expansion of the volume of liquid in the bulb. As this volume expands it forces the liquid up into the glass tube.

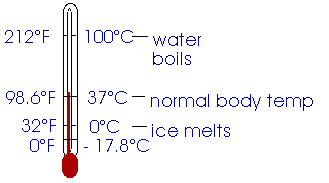
Heat is the transfer of energy due to a difference in temperature. If two objects are in contact with each other and no heat is transferred, we can say they are in thermal equilibrium.
After the First and Second Laws of Thermodynamics were established, it was realized that a basic statement about temperatures was important -- and even more fundamental. This statement is given the unusual name of the Zeroeth Law of Thermodynamics:
Two objects, each separately in thermal equilibrium with a third object, are in thermal equilibrium with each other.
Two objects in thermal equilibrium with each other are at the same temperature.
Return to Temperature ToC (c) Doug Davis, 2002; all rights reserved