
PHYsics 1151
Homework
Chapter 12; Temperature, Heat, and
Internal Energy
Ch 12: 2, 10, 29, 32, 40, 41, 47, 50, 57, 59, 61

| Return
to Calendar
|

12.2 If you have a fever of 102° on a Fahrenheit
thermometer, what is your temperature on a Celsius thermometer?
TC = [5/9]
[TF - 32°F ]
TC = [5/9] [102°F -
32°F ]
TC = [5/9] [70 F°
]
TC = [5 C°/9 F°]
[70 F° ]
TC = 39°C
12.10 On a concrete road, how large should the expansion
joints be between sections if each section is 15 m long. Consider a
temperature range of -10°C to + 35°C.
 l
=
l
= 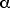 lo
lo  T
T
From Table 12.1, p 430, we find the coefficient of thermal
expansion for concrete to be
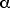 = 12 x 10-6 (C°)-1
= 12 x 10-6 (C°)-1
 l
= [12 x 10-6 (C°)-1] [15 m] [45
C°]
l
= [12 x 10-6 (C°)-1] [15 m] [45
C°]
 l
=8.1 x 10-3 m
l
=8.1 x 10-3 m
 l
= 8.1 mm
l
= 8.1 mm
12.29 A steel surveying tape is carefully calibrated at
20°C. However, it is used on a 38°C summer day. Are the
distances measured too large or too small? What is the percentage of
error introduced by this temperature change?
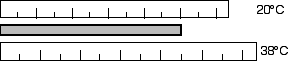
Due to the increase in temperature, the tape will elongate or get
longer. The length measured with the longer surveying tape will be
too small.
 l
=
l
= 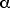 lo
lo  T
T
From Table 12.1, p 430, we find the coefficient of thermal
expansion for steel to be
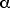 = 12 x 10-6 (C°)-1
= 12 x 10-6 (C°)-1
 l
= [12 x 10-6 (C°)-1] [ lo]
[18 C°]
l
= [12 x 10-6 (C°)-1] [ lo]
[18 C°]
 l
=2.16 x 10-4 lo = 2.2 x 10-4 lo
l
=2.16 x 10-4 lo = 2.2 x 10-4 lo
 l/lo
= .0022 = 0.22%
l/lo
= .0022 = 0.22%
(not a very big change, of course!)
12.32 If concrete roadway sections are poured butted against
each other when the temperature is 15°C, what will the thermal
stress be when the temperature reaches 40°C? Young’s
modulus for concrete is about 20 x 109 Pa.
The thermal stress is given by Equation 12.3
F/A = 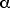 Y
Y  T
T
From Table 12.1, p 430, we have the coefficient of linear
expansion for concrete,
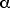 = 12 x 10-6 (C°)-1
= 12 x 10-6 (C°)-1
F/A = 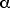 Y
Y  T
T
F/A = [12 x 10-6
(C°)-1] [20 x 109 Pa] [25
C°]
F/A = 6 x 106 Pa
12.40 A home furnace is rated at 50,000 Btu/h. What is this
power rating in kilowatts?
The conversion from Btu to J is given on p 437.
P = 50,000 Btu/h = 50,000
Btu/h [1054.8
J/Btu][h/3600 s] =
1.465 x 104 J/s
P = 1.465 x 104 J/s = 14.65 x
103 J/s
[W/(J/s)] = 14.65 x 103 W =
14.67 kW
12.41. A child’s wading pool contains 1.2 m3
of water at 15°C. How much heat must be added to the pool to
bring the temperature to 27°C?
Equation 12.5 gives us the heat needed -- but in terms of the
mass of the water. What is the mass of 1.2
m3 of water?
m = 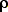 V = (1000 kg/m3)(1.2 m3) = 1.2 x 103
kg = 1,200 kg
V = (1000 kg/m3)(1.2 m3) = 1.2 x 103
kg = 1,200 kg
 Q
= c m
Q
= c m  T
T
 Q
= [4186 J/kg C°] [1,200 kg] [12
C°]
Q
= [4186 J/kg C°] [1,200 kg] [12
C°]
 Q
=6.03 x 107 J
Q
=6.03 x 107 J
12.47 Once its melting point is reached, how much heat is
required to melt a 0.5 kg bar of gold?
From Table 12.3, p 440, the Heat of Fusion for gold is Lf
= 0.644 x 105 J/kg
Q = Lf m
Q = [ 0.644 x 105 J/kg ]
[ 0.5 kg ] = 0.322 x 105 J = 3.22 x 104
J = 32,200 J
12.50 If 0.60 kg of boiling hot coffee is poured into a
0.250-kg steel camping cup initially at 20°C, what is the final
temperature of the cup-coffee system?
Q1 + Q2 = 0
Q1 = Qcup = ccup mcup  Tcup
= csteel msteel
Tcup
= csteel msteel  Tsteel
Tsteel
From Table 12.2, p 438, we find that the specific heat of steel is
csteel = 450 J/kg C°
Q1 = csteel msteel  Tsteel
= ( 450 J/kg C° ) (0.250 kg) (Tf - 20°C)
Tsteel
= ( 450 J/kg C° ) (0.250 kg) (Tf - 20°C)
Q2 = Qcoffee = ccoffee mcoffee  Tcoffee
= cwater mwater
Tcoffee
= cwater mwater  Twater
Twater
Q2 = cwater mwater  Twater
= (4186 J/kg C°) (0.60 kg) (Tf - 100°C)
Twater
= (4186 J/kg C°) (0.60 kg) (Tf - 100°C)
where we have taken the specific heat of coffee to be the same as
the specific heat of water,
ccoffee = cwater = 4186 J/kg
C°.
Now we can put these all together as
Q1 + Q2 = 0
( 450 J/kg C° ) (0.250 kg) (Tf
- 20°C) + (4186 J/kg C°) (0.60 kg)
(Tf - 100°C) = 0
112.5 (J/C°) Tf - 2,250 J +
2,512(J/C°) Tf - 251,200 J =
0
112.5 (J/C°) Tf +
2,512(J/C°) Tf = 2,250 J +
251,200 J
[ 112.5 + 2,512 ](J/C°)
Tf = [ 2,250 + 251,200 ] J
2,624.5 Tf = 253,450 °C
Tf = 253,450 °C/2,624.5
Tf = 96.6°C
12.57 A 0.050-kg ice cube, initially at - 5.0°C, is
placed in 0.30 kg of water at 25°C. What is the final
temperature of the water? Or, if melting is not complete, how much
ice remains unmelted at thermal equilibrium? Assume no heat is lost
to the environment.
First, assume all the ice melts, so Tf > 0°C
Q1 + Q2 = 0
Q1 = heat lost by the water
Q1 = cwater mwater  Twater
= (4186 J/kg C°)(0.30 kg)(Tf - 25°C)
Twater
= (4186 J/kg C°)(0.30 kg)(Tf - 25°C)
Q1 = (4186 J/kg C°)(0.30
kg)(Tf - 25°C) = 1,256
(J/C°) Tf - 31,395 J
Q2 = heat gained by ice
Q2 = heat gained in raising ice to 0°C +
+ heat gained in melting ice +
+ heat gained in raising the melted ice (now water) to temperature
Tf
Q2 = cice mice  T1
+ Lf,ice mice + cwater mice
T1
+ Lf,ice mice + cwater mice  T2
T2
Q2 = [2090 J/kg
C°][0.05 kg][0°C - (- 5°C)]
+
+ [3.33 x 105 J/kg][0.05
kg] +
+ [4186 J/kg C°][0.05
kg][Tf - 0°C]
Be very careful with these temperature changes!
Q2 = 522.5 J + 16,650 J + 209.3
(J/C°) Tf - 0
Q2 = 17,172.5 J + 209.3 (J/C°)
Tf
Now we are ready to go back to
Q1 + Q2 = 0
1,256 (J/C°) Tf - 31,395 J +
17,172.5 J + 209.3 (J/C°) Tf =
0
1,256 (J/C°) Tf + 209.3
(J/C°) Tf = 31,395 J -
17,172.5 J
1,465 (J/C°) Tf = 14,222 J
Tf =
[14,222/1,465]°C
Tf = 9.7°C
And this is a reasonable answer.
12.59 A 0.15-kg ice cube initially at -15°C is placed in
0.30 kg of water at 25°C. What is the final temperature of the
water? Or, if melting is not complete, how much ice remains unmelted
at thermal equilibrium? Assume no heat is lost to the
environment.
This should look very similar to problem 12.57. Now we have a larger
and colder ice cube.
First, assume all the ice melts, so Tf > 0°C
Q1 + Q2 = 0
Q1 = heat lost by the water
Q1 = cwater mwater  Twater
= (4186 J/kg C°)(0.30 kg)(Tf - 25°C)
Twater
= (4186 J/kg C°)(0.30 kg)(Tf - 25°C)
Q1 = (4186 J/kg C°)(0.30
kg)(Tf - 25°C) = 1,256
(J/C°) Tf - 31,395 J
Q2 = heat gained by ice
Q2 = heat gained in raising ice to 0°C +
+ heat gained in melting ice +
+ heat gained in raising the melted ice (now water) to temperature
Tf
Q2 = cice mice  T1
+ Lf,ice mice + cwater mice
T1
+ Lf,ice mice + cwater mice  T2
T2
Q2 = [2090 J/kg
C°][0.15 kg][0°C - (- 15°C)]
+
+ [3.33 x 105 J/kg][0.15
kg] +
+ [4186 J/kg C°][0.15
kg][Tf - 0°C]
Be very careful with these temperature changes!
Q2 = 4,702 J + 49,950 J +
628(J/C°)Tf - 0
Q2 = 54,652 J +
628(J/C°)Tf
Q1 + Q2 = 0
1,256 (J/C°) Tf - 31,395 J +
54,652 J + 628 (J/C°)Tf = 0
[1,256 + 628](J/C°)Tf +
[- 31,395 + 54,652]J = 0
1,884](J/C°)Tf + 23,257J =
0
1,884](J/C°)Tf = -
23,257J
Tf = [-
23,257/1,884]°C
Tf = - 12.3°C
And this is inconsistent with our assumption that
Tf > 0. Therefore, we have to look at another
possibility. This time, we shall assume Tf = 0°C and
only mmelt of the ice melts. Our calculation for
Q1 is almost the same as before but our calculation for
Q2 is quite different.
Q1 = heat lost by the water
Q1 = cwater mwater  Twater
= (4186 J/kg C°)(0.30 kg)(0°C - 25°C)
Twater
= (4186 J/kg C°)(0.30 kg)(0°C - 25°C)
Q1 = (4186 J/kg C°)(0.30 kg)(-
25°C) = - 31,395 J
Q2 = heat gained by ice
Q2 = heat gained in raising ice to 0°C + heat gained
in melting some ice
Q2 = cice mice  T1
+ Lf,ice mmelt
T1
+ Lf,ice mmelt
Q2 = [2090 J/kg
C°][0.15 kg][0°C - (- 15°C)]
+ [3.33 x 105
J/kg][mmelt]
Q2 = 4,702 J + 3.33 x 105
(J/kg)mmelt
Q1 + Q2 = 0
- 31,395 J + 4,702 J + 3.33 x 105
(J/kg)mmelt = 0
- 26,693 J + 3.33 x 105
(J/kg)mmelt = 0
3.33 x 105 (J/kg)mmelt =
26,693 J
mmelt = [26,693/333,000]
kg
mmelt = 0.080 kg
Since we started with 0.150 kg, this is a reasonable answer. There
will remain 0.070 kg of ice that has not melted.
12.61 The inside diameter of a steel lid and the outside
diameter of a glass peanut butter jar are both exactly 11.50 cm at
room temperature, 21.0°C. If the lid is stuck and you run
80°C hot water over it until the lid and the jar top both come
to 80°C, what will the new diameters be?
For the steel lid, the change in the diameter,  d,
is
d,
is
 d
=
d
= 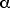 do
do  T
= [12 x 10- 6(C°)-1] [11.50 cm] [59
C°] = 0.0081 cm
T
= [12 x 10- 6(C°)-1] [11.50 cm] [59
C°] = 0.0081 cm
so the new diameter is
dnew = do +  d
= 11.5081 cm
d
= 11.5081 cm
For the glass jar, the change in the diameter, Dd, is
 d
=
d
= 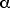 do
do  T
= [10 x 10- 6(C°)-1] [11.50 cm] [59
C°] = 0.0068 cm
T
= [10 x 10- 6(C°)-1] [11.50 cm] [59
C°] = 0.0068 cm
so the new diameter is
dnew = do +  d
= 11.5068 cm
d
= 11.5068 cm
Now the difference in the two diameters is 0.0013 cm. That
may be enough to break the lid loose and let us make our
peanut butter sandwich!

| Return
to Calendar
|

(c)2000, Doug Davis; all rights
reserved



![]() T1
+ Lf,ice mice + cwater mice
T1
+ Lf,ice mice + cwater mice ![]() T2
T2