
Work Done by a Gas
![]() When
a gas expands it does work on its surroundings. That work is equal
to the area under the curve on a P-V diagram which describes that
expansion.
When
a gas expands it does work on its surroundings. That work is equal
to the area under the curve on a P-V diagram which describes that
expansion.
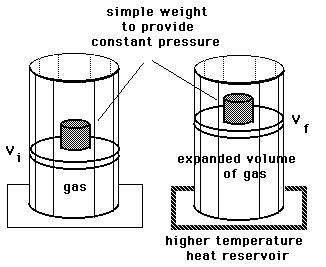
Isobaric process:
Process at constant pressure:
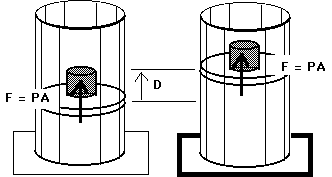
W = F D
W = ( F / A ) ( A D )
W = P ( AD )
W = P (![]() V)
V)
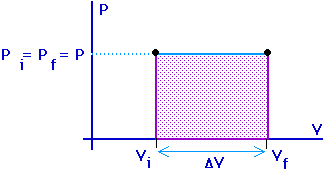
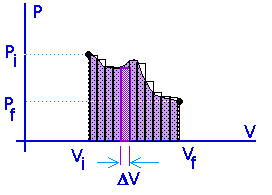
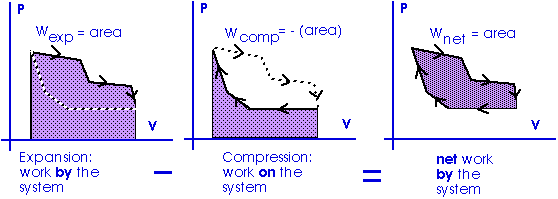
Work = area under the curve on a P-V diagram.
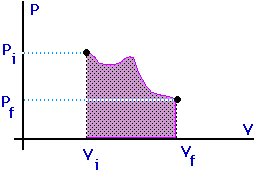
Work = area under the curve on a P-V diagram -- even if the process is not isobaric.
Isometric processes:
Isochoric:
Constant volume:
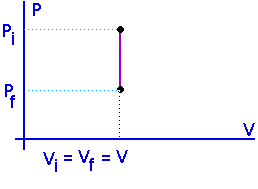
No work is done in an isometric process.
Isothermal process:
Process at constant temperature:
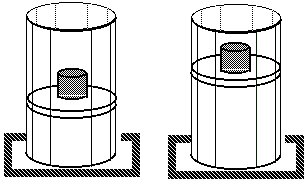
We can keep the temperature constant by having the system in contact with a heat reservoir.
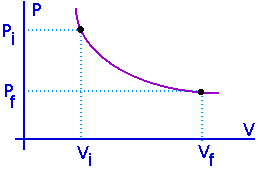

Adiabatic process:
Process with zero heat flow:
Insulated:
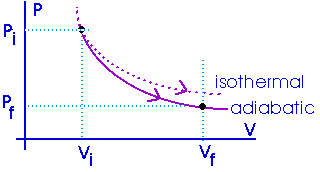
The amount of work done will be less than for an isothermal process between the same two volumes. Notice that an adiabat is steeper than an isotherm.
Example:
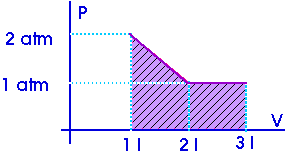
W = 2.5 atm-l
Now, what is an "atmosphere-liter"? It is a pressure (atm) multiplied by a volume (liter) so it must be some sort of work or energy. Now, this is just a units conversion question,
W = 253 Pa-m3 [ (N/m2) / Pa ]
W = 253 N-m
W = 253 J
|
|
|
|
|
|
|
|
||
|
|
|||