
Kinetic Theory of Gases
![]() Pressure
is caused by the constant bombardment of the many individual
molecules of a gas.
Pressure
is caused by the constant bombardment of the many individual
molecules of a gas.
![]() The
ideal gas law can be explained by the constant bombardment of the
many individual molecules of a gas.
The
ideal gas law can be explained by the constant bombardment of the
many individual molecules of a gas.
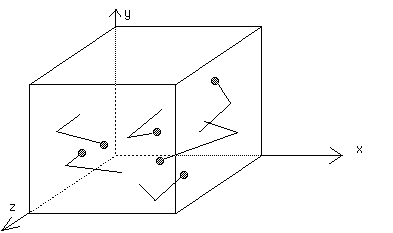
Pressure is caused by the collisions of gas molecules with the walls of the container holding the gas.
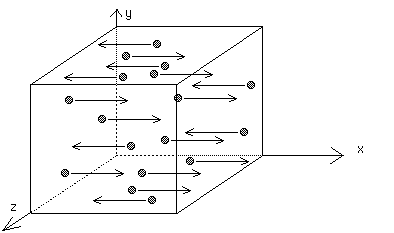
Assume one-third of the molecules move along the x-axis at constant speed v.
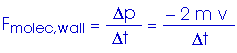
![]()
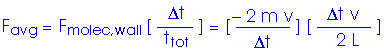
![]()
This is the average force exerted by each single molecule. But we have N/3 molecules hitting this wall. Therefore, the total force on the wall is
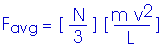
Now, to get the pressure. Remember, in our simplified model, we are looking at a cube with edge length L,
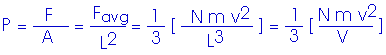
P V = (1/3) N m v2
The kinetic energy of each molecule is KE = (1/2) m v2 so we can rewrite this as
We already know that
so this means
or
Let us replace our constant speed v with an average speed <v>,
(1/2) m <v>2 = Eint
Eint, tot = N (3/2) k T
Eint, tot = (3/2) N k T = (3/2) n R T
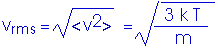
|
|
|
|
|
|
|
|
||
|
|
|||