
The First Law of Thermodynamics
![]() The
change in internal energy
The
change in internal energy ![]() U
of a system is equal to the heat that flows into the system Q
minus the work W done by the system on its surroundings;
U
of a system is equal to the heat that flows into the system Q
minus the work W done by the system on its surroundings;
![]() U
= Q - W; this is known as the First Law of
Thermodynamics.
U
= Q - W; this is known as the First Law of
Thermodynamics.
![]() The
First Law of Thermodynamics is a restatement of the Work-Energy
Theorem and the ideas of Conservation of
Energy.
The
First Law of Thermodynamics is a restatement of the Work-Energy
Theorem and the ideas of Conservation of
Energy.
Example:
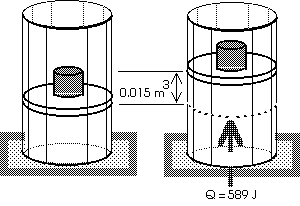
Example:
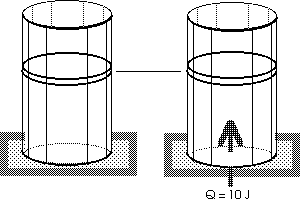
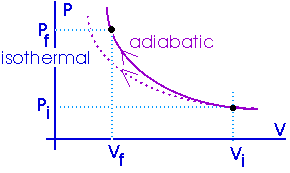
|
|
|
|
|
|
|
|
||
|
|
|||