
PHY 1151
Spring 2000
Fourth Hour Exam
April 26, 2000
![]()
![]()

PHY 1151
Spring 2000
Fourth Hour Exam
April 26, 2000
![]()

![]()
1. A roasted turkey cools from 85°C to 80°C in 10 min when sitting in a 25°C room. How long does it require to cool from 85°C to 55°C?
This is homework question 13.30.This requires an application of Newton’s Law of Cooling, equation 13.3,
T(t) = Tsur + T e - t/
T is the initial temperature difference of the turkey and its surroundings;
T = 85°C - 25°C = 60 C°
Knowing that it cools from 85°C to 80°C allows us to solve for the “time constant”
in this equation;
Now we know the time constantand we can use Newton’s Law of Cooling to go back and solve for t, the time, when T, the temperature, is 55°C.
t = 78 min
What will its pressure be when the temperature is increased to 50°C?
This is homework question 14.1.The ideal gas law is
PV = nRT Being in a rigid container, the gas’ volume does not change; V = Vo = constant. That means we can use the ideal gas law as
T/P = To/Po
T/P = To/Poor
T = P [ To/Po ] = [ P/Po ] To
T = P [ To/Po ] = [ P/Po ] ToRemember, these temperatures must be absolute temperatures,
To = 25°C = 298 K
T = [ P/Po ] To = [2.0 atm/1.0 atm] [298 K] = [ 2 ] [ 298 K] = 596 K
T = 596 K = (596 - 273)°C = 323°C = T
Or, while V = const, we can write the ideal gas law as
P/T = Po/To
or
P = T [ Po/To ] = [ T/To ] Po
Remember, these temperatures must be absolute temperatures,
To = 25°C = 298 K and
T = 50°C = 323 K
P = [ T/To ] Po
P = [ 323 K/298 K ] (1 atm)
P = 1.08 atm
3. An air track glider has a mass of m =
0.200 kg and is attached to spring which have an effective spring
constant of k = 8.0 N/m. It oscillates with an amplitude of A = 0.10
m.
a) What is the total energy of this simple harmonic
oscillator?
b) What is the speed of the glider as it passes through
equilibrium?
c) What is the period of this simple harmonic
oscillator?
This problem is similar to the SHM and Energy Conservation lab that we did.
4. A train whistle sounds at 500 Hz. What frequency is heard by a stationary observer when the train approaches at 25 m/s? Use v = 340 m/s as the speed of sound in air.
This is one part of homework question 16.49 . It requires use of Equation 16.17 or 16.19. Be careful with the sign conventions; since the source is approaching, that means vx < 0 .
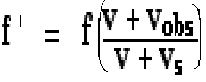
![]()
f ' = (500 Hz) (1.079) = 540 Hz
Always ask if an answer is reasonable. If the frequency of the stationary train is 500 Hz and you calculate a frequency of 37 Hz or 0.73 Hz or something like that, alarms should go off! What do you expect to hear!?!?! Do you expect to hear the moving train? Of course you do! Can you hear frequencies of 37 Hz or 0.73 Hz? No!
![]()
(C) 2000, Doug Davis; all rights reserved