
The First Law of Thermodynamics
The change in internal energy ![]() U
of a system is equal to the heat that flows into the system Q minus the work
W done by the system on its surroundings;
U
of a system is equal to the heat that flows into the system Q minus the work
W done by the system on its surroundings;
![]() U
= Q - W
U
= Q - W
this is known as the First Law of Thermodynamics.
The First Law of Thermodynamics is a restatement of the Work-Energy Theorem and the ideas of Conservation of Energy.
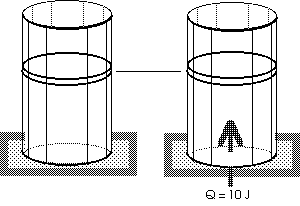
Example:
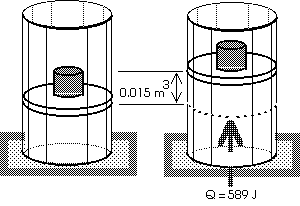
Example:
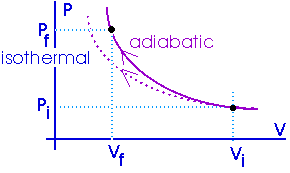
|
|
|
|
|
|
|
|
||
|
|
|||