
Ideal Heat Engines
![]() Heat
engines convert internal energy to mechanical energy.
Heat
engines convert internal energy to mechanical energy.
![]() The
operation of a reversible heat engine can be described on a PV
diagram.
The
operation of a reversible heat engine can be described on a PV
diagram.
![]() The
efficiency of a reversible heat engine depends upon the
temperatures between which it operates.
The
efficiency of a reversible heat engine depends upon the
temperatures between which it operates.
![]() We
will describe a heat engine with a diagram like this:
We
will describe a heat engine with a diagram like this:
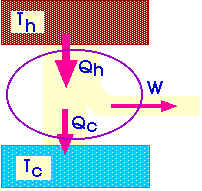
Qh = Qc + W
The efficiency of a heat engine describes how efficiently it turns heat into work.
Sadi Carnot
Carnot's Principle: An irreversible heat engine operating between two heat reservoirs at constant temperatures cannot have an efficiency greater than that of a reversible heat engine operating between the same two temperatures.
Corollary: All reversible heat engines operating between the same temperatures have the same efficiency.
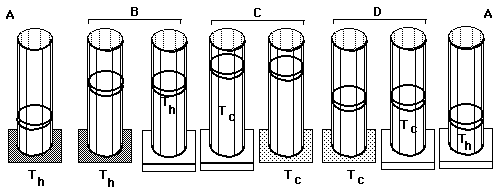
Carnot's Cycle:
Carnot's reversible "engine" uses isothermal and adiabatic processes between two heat reserviors at temperatures Th (hot) and Tc (cold).
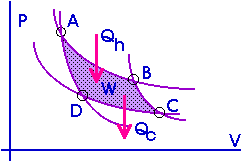
A Carnot cycle can also be represented on a PV diagram.
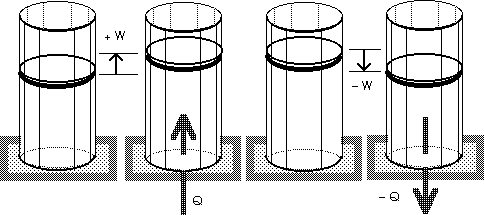
A refrigerator is a heat engine, run in
reverse.
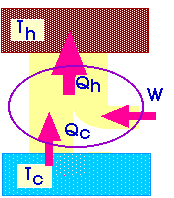
W + Qc = Qh
Another form of the Second Law of Thermodynamics is
that
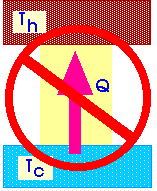
It is not possible to make a heat engine whose only effect is to absorb heat from a high-temperature region and turn all that heat into work.
That is, it is not possible to design a heat engine that does not exhaust heat to the environment.
Or, it is not possible to design a heat engine that has an efficiency of 1.00 or 100%.
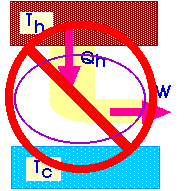
If we could design such a 100% efficient heat engine, we could then use that heat engine to power a refrigerator. And the net result of that combination would be to cause heat to flow from a cold temperature to a high temperature.
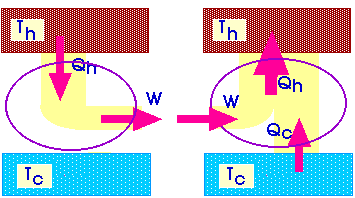
And that gets us back to our original statement of the Second Law of Thermodynamics:
|
|
|
|
|
|
|
|
||
|
|
|||