
Change of State
Crystalline materials change phase -- melt and freeze or vaporize and condense -- at a single, fixed temperature.
Heat is required for a change of phase of a substance. The ratio of the heat to the mass of the substance involved is called the latent heat of the substance.
Latent heat of fusion describes the heat necessary to melt (or freeze) a unit mass of a substance.
Latent heat of vaporization describes the heat necessary to vaporize (or condense) a unit mass of a substance.
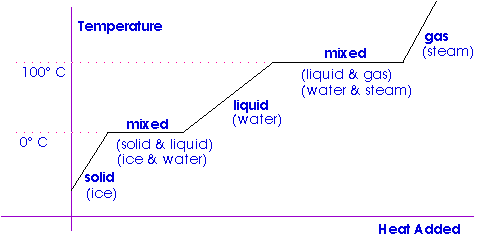
We have already seen
For a change of state, we now have
and
|
|
|
|
|
|
|
|
||
|
|
|||