
Summary
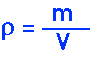
![]()
![]()
Fbouyant =
wliquid
Several different
systems of units are in common useage when dealing with
pressure:
1 pascal = 1 Pa = 1 [ N /
m2 ]
1 atm = 1.013 x 105
Pa
1 bar = 100 kPa = 1 x 10
5 Pa
1 atm = 1.013
bar
1 atm = 101.3
kPa
1 mm Hg = 133
Pa
1 atm = 760 mm
Hg
1 Torr = 1 mm
Hg
1 atm = 760
Torr
(Units of pounds per square inch (PSI) are also commonly used but we will avoid those in this course).
For many situations -- for most situations involving a liquid but not a gas -- we can assume the density does not change. This is called an incompressible fluid. For those cases,
(1/2)
or
If the vertical height y does not change, this means
(1/2) ![]() v2
+ P = constant
v2
+ P = constant
or
|
|
|
|
|
|
|
|
||
|
|
|||