Since igneous rocks form at high temperatures, and under pressure conditions ranging from one to several atmospheres. However, the conditions at the Earth's surface are somewhat different than the conditions at which most rocks and minerals form. Therefore, the materials are no longer at equilibrium when they are exposed to surface conditions. Under these conditions, there is a tendency for all ordered systems to seek lower levels of energy or order. This is all done through weathering.
Weathering - the disintegration and decomposition of rock at or near the surface of the earth. It affects the rocks in place and no transport is involved. This distinguishes weathering from erosion.
Mechanical/physical weathering - physical disintegration of a rock into smaller fragments, each with the same properties as the original. Occurs mainly by temperature and pressure changes.
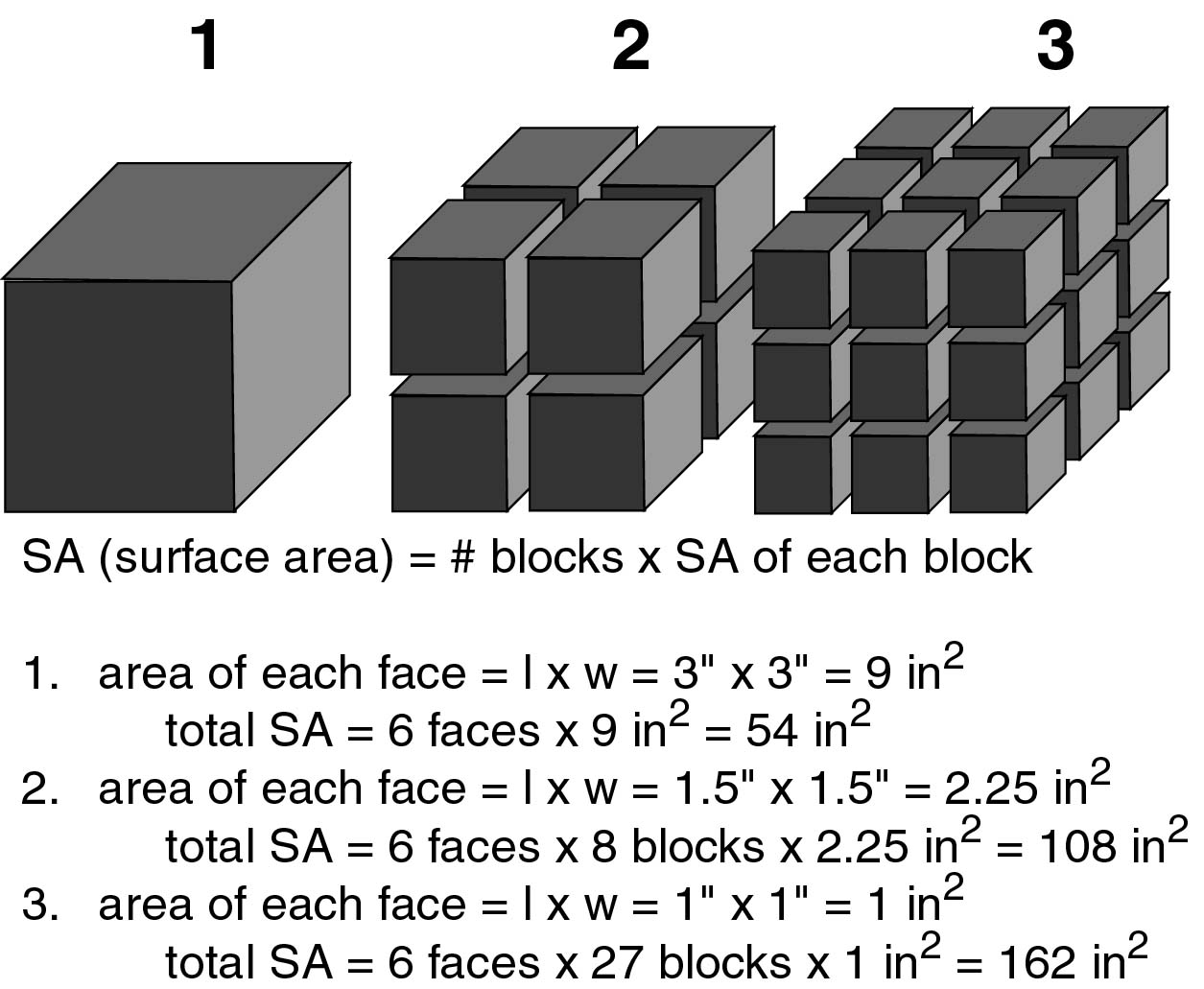
Chemical weathering - process by which the internal structure of a mineral is altered by the addition or removal of elements. Change in phase (mineral type) and composition are due to the action of chemical agents. Chemical weathering is dependent on available surface for reaction temperature and presence of chemically active fluids. Smaller particle sizes weather by chemical means more rapidly than large particles due to an increase of surface area. Look at the diagram below and you will see that as the particles get smaller, the total surface area available for chemical weathering increases.

Erosion - the incorporation and transportation of weathering products by a mobile agent such as wind, water, ice.
All three processes may act independently, but will more often than not, occur simultaneously. Different circumstance will have one weathering process more important than another. The processes may also act in concert with one another.
Types of Mechanical Weathering:
 |
Frost Wedging - water expands when it freezes. This photograph shows the individual layers within the sedimentary rock breaking apart through repeated cycles of freeze-thaw. A similar process happens when the rock is repeatedly wetted and dried as salt crystals dissolve from the rock then grow when it is dried. Both processes can result in the rocks being heaved - so what was once a nice regular pattern of bricks set in a pavement will eventually become a chaotic jumble of bricks oriented every which way. Thermal Expansion and Contraction - heating causes rock to expand, cooling results in contraction; different minerals expand and contract at different rates. This phenomena will look very similar to frost wedging and salt crystal growth, but will typically happen in climates that undergo extreme diurnal temperature changes. |
 |
Mechanical Exfoliation - rock breaks apart in layers that are parallel to the earth's surface; as rock is uncovered, it expands (due to the lower confining pressure) resulting in exfoliation. The photograph is from G. K. Gilbert (1903) in Sequoia National Park. The granite boulder is shaped by exfoliation; the boulder is about 40 feet in diameter, and the separated fragment resting on it is about 10 feet thick. Exfoliation is very common whenever plutonic igneous rocks are exposed. Since the plutonic rocks cool at depth under great pressure, they essentially de-pressurizes once the overburden is removed. This causes sheets of rock to peel off subparallel to the earth's surface, or whatever is the least pressurized surface. |
 |
In this photo from Yosemite National Park, the exfoliation sheets are subparallel to the valley walls. |
 |
Abrasion - physical grinding of rock fragments. Here, the photo shows some pits that have been eroded into the rock by sandblasting. Along with the physical weathering (the sandblasting), chemical weathering has taken place as the rock shows some signs of solution weathering as well. |
 |
Another photograph which shows the powerful effect of wind generated abrasion is the Double Arch from Arches National Park. The edges of the arches have weathered along joints, preexisting tectonically controlled vertical surfaces in the rock. Then mechanical abrasion took over and carved out the arches. |
Types of Chemical Weathering:
 Dissolution
Dissolution
H2O + CO2 + CaCO3 --> Ca+2 + 2HCO3-Dissolution is very common in areas that have a great deal of limestone. Acidic waters (from pollution or natural) dissolve limestone allowing for additional water to gain entrance. Can cause sinkholes and karst features as well as dissolution of statutes and grave stones.
water + carbon dioxide + calcite dissolve into calcium ion and bicarbonate ion
Oxidation (rust)
4Fe+2 +3O2 --> 2Fe2O3Hydrolysis
ferrous iron + oxygen combine to form ferric iron oxide (hematite)Will happen to all iron-bearing silicates to varying degrees. Common reaction minerals are hematite, limonite, and goethite.
2KAlSi3O8 + 3H20 --> Al2Si2O5(OH)4 + 4SiO2 + 2K(OH)
potassium feldspar in acidic water hydrolyses to kaolinite + quartz + potassium hydroxideSilicate minerals (unstable at the earth's surface) weather to form clay minerals such as kaolinite (stable at the earth's surface). Feldspars typically weather to produce clay minerals.
Factors that influence chemical weathering
Climate
Living Organisms
bioturbation
acid production and mineral
decomposition
Time
Mineral composition
Goldich Dissolution Series
(Bowen's Reaction Series)
Chemical Weathering Products
Clays
Metals ores
Rounding of boulders (chemical
exfoliation)
Soils and Soil Formation
Dependence of weathering type on the mean temperature and annual rainfall.
Weathering rates depend on the composition of the rock, temperature range and rainfall amount. Weathering produces soils. Soils may or may not remain in place, and any soil may be a combination of residual and transported material.
Residual soil: Remains in place; has not been transported (gruss).
Transported soil: Transported by wind or
water
and deposited.
A complete soil profile will have the following components:
O horizon: Organic debris and leaf litter on the surface.A horizon: Topsoil - leaching, water movement down, Organic and Mineral material transported downward.
B horizon: Subsoil - accumulation of dissolved material and fine clays, hardpan.
C horizon: Partially altered parent rock material.
Bedrock: Unweathered parent rock material.
These horizons are not present in all soil profiles.
In areas of rapid erosion, B & C may be present or C only. In some
areas no soil profile will develop at all.
Factors in Soil Formation
Mineral stability
Sediments are the by-product of weathering. Sediments are particles of minerals, some of them altered from the original rock, some simply reduced in sized, and some new minerals by reaction. The stability of minerals can be predicted using the Bowen's reaction series, however, in the case of the weathering series this is known as the Goldich Dissolution Series:
Olivine
Mg Pyroxene Calcic Plagioclase
Mg-Ca Pyroxene Calcic-Alkalic Plagioclase
Amphibole Alkalic-Calcic Plagioclase
Biotite Alkalic Plagioclase
Potassium Feldspar
Muscovite
Quartz
Minerals crystallize from a melt at different temperatures during the migration and emplacement of the magma. Those minerals that crystallize at higher temperatures will be the least stable at the surface. From this it is obvious that quartz will be the most stable mineral in the weathering environment, and will be a dominant constituent of sediments and sedimentary rocks.